 About Authors:
About Authors:
Manish Jaimini*, Vishal Joshi
Dept. Of Pharmaceutics, Jaipur college of Pharmacy,
Sitapura, Jaipur (Raj.) 302022
*manishjaimini@gmail.com
ABSTRACT
Novel drug delivery system as a major advance to solving the problems related to the release of the drug at specific site. These systems have several advantages over conventional multi dose therapy. There are various approaches in delivering a therapeutic substance to the target site in a sustained release fashion. One such approach is using microspheres as carriers for drugs. Microencapsulation is used to modify and delay drug release from pharmaceutical dosage forms. Microspheres efficiently utilized in controlled delivery of many drugs but wastage of drug due to low drug entrapment efficiency is the major drawback of such micro-particulate system. This review provides brief information about floating microspheres, method of preparations, evaluation and application of microspheres for sustained drug delivery.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1537
Introduction 1
In recent years, in oral sustained or controlled release multiparticulate drug delivery system extensive research works have been occurs because of its advantages over monolithic dosage form. Now a day’s floating concept of multiparticulate reservoir type delivery system more importance.
Oral sustained release floating multiparticulate drug delivery system include low density floating micro pellets, floating micro beads (acrylic resin based), hollow microspheres (micro balloons) etc. The article published on the development of both effervescent and non-effervescent type of floating drug delivery. Much research has been focused and the scientists are still exploring the field of hollow microspheres.1
Mechanism of floating microspheres
The microspheres come to contact with gastric fluid and gel formers, polysaccharides, and polymers hydrate to form a colloidal gel barrier that controls the rate of fluid penetration into the device and consequent drug release. As the exterior surface of the dosage form dissolves, the gel layer is maintained by the hydration of the adjacent hydrocolloid layer. The air trapped by the swollen polymer lowers the density and confers buoyancy to the microspheres. However a minimal gastric content needed to allow proper achievement of buoyancy. Hollow microspheres of acrylic resins, eudragit, polyethylene oxide, ethyl cellulose. cellulose acetate; polystyrene floatable shells; polycarbonate floating balloons and gelucire floating granules are the recent developments.2
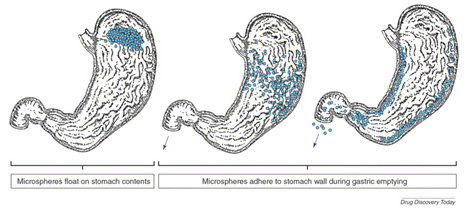
Figure 1 Floating of microspheres in stomach
[adsense:468x15:2204050025]
Mechanism of drug release from the microspheres
The mechanism of drug release from multiparticulates can occur in the following ways:
Diffusion: On contact with aqueous fluids in the gastrointestinal tract (GIT), water diffuses into the interior of the particle. Drug dissolution occurs and the drug solutions diffuse across the release coat to the exterior.
Erosion: Some coatings can be designed to erode gradually with time, thereby releasing the drug contained within the particle.
Osmosis: In allowing water to enter under the right circumstances, an osmotic pressure can be built up within the interior of the particle. The drug is forced out of the particle into the exterior through the coating3
Methods of preparation of floating microspheres 64-69
1. Solvent evaporation method
Floating multiparticulate dosage form was prepared by solvent diffusion and evaporation methods to create the hollow inner core. The polymer is dissolved in an organic solvent and the drug is either dissolved or dispersed in the polymer solution. The solution containing the drug is then emulsified into an aqueous phase containing polyvinyl alcohol to form oil in water emulsion. After the formation of a stable emulsion, the organic solvent is evaporated either by increasing the temperature under pressure or by continuous stirring6, the solvent removal leads to polymer precipitation at the o/w interface of droplets, forming cavity and thus making them hollow to impart the floating properties4.
a. Oil-in-oil emulsion solvent evaporation method
During a great number of microencapsulation techniques for the formation of sustained release drug delivery systems, one of the popular methods is the emulsion solvent evaporation method. In order to increase the encapsulation efficiency, a mixed solvent system comprising 1:1 proportions of Acetonitrile and dichloromethane was used as a dispersed phase, and the corn oil was used as a continuous phase. Microspheres containing anti-hypertension drug, Felodipine, were prepared by the emulsion solvent evaporation method (o/o) using Acrylate methacrylate copolymers. The morphology of the microspheres was evaluated using scanning electron microscope, which showed a spherical shape with smooth surface5.
b. Foam-based method for floating microparticles
A novel multi-particulate gastro retentive drug delivery system based on low-density foam powder has been proposed in which, The drug and release-rate-controlling polymer were dissolved in Methylene chloride. Polypropylene foam powder was then dispersed within this organic phase. The resulting suspension was subsequently emulsified into an external aqueous Poly (vinyl alcohol) solution and agitated with a stirrer to allow microparticle formation. The microparticles were separated by being sieved, washed with water and dried in a desiccator; they were irregular in shape and highly porous. Importantly, the drug encapsulation efficiency was high and almost independent of the theoretical loading of the system. In all cases, good in-vitro floating behavior was observed. Interestingly, a broad spectrum of release patterns could be obtained with the investigated formulations6.
2. Ionotropic gelation method 7, 8
Ionotropic gelation is based on the ability of Polyelectrolytes to cross link in the presence of counterions to form beads. Since, the use of Alginates, Gellan gum, Chitosan and Carboxymethyl cellulose for the encapsulation of drug and even cells, ionotropic gelation technique has been widely used for this purpose. The natural polyelectrolytes inspite, having property of coating on the drug core and acts as release rate retardants contains certain anions on their chemical structure. These anions forms meshwork structure by combining with the polyvalent cations and induce gelation by binding mainly to the anion blocks. The hydro gel beads are produced by dropping a drug-loaded polymeric solution into the aqueous solution of polyvalent cations.
3. Emulsion solvent diffusion method 5,9
Kawashima and colleagues5,6 proposed hollow microspheres with drug in their outer polymer shell prepared by novel emulsion solvent diffusion method. Based on Eudragit-S (an enteric polymer), containing the drug in the polymeric shell. The solution of polymer and drug in ethanol methylene chloride is poured into an agitated aqueous solution of poly (vinyl alcohol). The ethanol rapidly partitions into the external aqueous phase and the polymer precipitates around methylene chloride droplets. The subsequent evaporation of the entrapped methylene chloride leads to the formation of internal cavities within the microparticles.
4. List of polymers used in hollow microspheres 10,11
Cellulose acetate, Ethyl Cellulose , Chitosan, Eudragit, Acrycoat, Methocil, Polyacrylates, Polyvinyl acetate, Carbopol, Agar, Polyethylene oxide, Polycarbonates, Acrylic resins and Polyethylene oxide.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Evaluation parameters of floating microspheres
1. Micromeritics properties 12,13
Floating microspheres are characterized by their micromeritics properties such as particle size, Flow property and Density. Angle of Repose 13,14Hausner’s Ratio, compressibility index is determined by measuring the change in volume using a bulk density apparatus; angle of repose is determined by fixed funnel method. The hollow nature of microspheres is confirmed by scanning electron microscopy.
2. Floating behavior14
Appropriate quantity of the floating microspheres were placed in 100 ml of the simulated gastric fluid (SGF, pH 2.0), the mixture was stirred with a magnetic stirrer. The layer of buoyant microparticulate was pipetted and separated by filtration. Particles in the sinking particulate layer were separated by filtration. Particles of both types were dried in a desiccator until constant weight was achieved. Both the fractions of microspheres were weighed and buoyancy was determined by the weight ratio of floating particles to the sum of floating and sinking particles.
Buoyancy (%) = Wf / Wf + Ws
Where, Wf and Ws are the weights of the floating and settled microparticles
3.% Drug entrapment 15
Accurately weighed microspheres were taken, thoroughly triturated and suspended in a minimal amount of solvent. The suspension was filtered to separate shell fragments. Drug contents were analyzed and % Drug entrapment is calculated by using following equation.
% Drug Entrapment= Actual drug content /Theoretical drug content×100
4. In-vitro release studies15
The release rate of floating microparticulate was determined in dissolution apparatus. A weighed amount of floating microspheres equivalent to Dose of drug is taken and placed in the basket type of dissolution test apparatus. The dissolution fluid was maintained at 37 ± 1°C at a rotation speed. Perfect sink conditions prevailed during the drug release study.
5. In-vivo studies 16
The in-vivo floating behavior can be investigated by X-ray photography of hollow microparticulate loaded with Barium sulphate in the stomach of beagle dogs. The in vitro drug release studies are performed in a dissolution test in a dissolution media. The in-vivo plasma profile can be obtained by performing the study in suitable animal models.
Advantages of floating microspheres 16,17
1. Improves patient compliance by decreasing dosing frequency.
2. Bioavailability enhances despite first pass effect because fluctuations in plasma drug concentration are avoided; a desirable plasma drug concentration is maintained by continuous drug release.
3. Better therapeutic effect of short half-life drugs can be achieved.
4. Gastric retention time is increased because of buoyancy.
5. Drug releases in controlled manner for prolonged period.
6. Site-specific drug delivery to stomach can be achieved.
7. Enhanced absorption of drugs which solubilise only in stomach.
8. Superior to single unit floating dosage forms as such microspheres releases drug uniformly and there is no risk of dose dumping.
9. Avoidance of gastric irritation, because of sustained release effect, floatability and uniform release of drug through multiparticulate system.
Disadvantages
Floating system is not feasible for those drugs that have solubility or stability problem in GIT.
1. These systems require a sufficiently high level of fluids in the stomach for enabling the system to float and to work efficiently.
2. The drugs that are significantly absorbed throughout gastrointestinal tract, which undergo extensive first pass metabolism, may not be suitable for FDDS as the slow gastric emptying limits the systemic bioavailability.
3. Some drugs present in the floating systems cause irritation to gastric mucosa.18,19,20
Applications of floating microspheres
Sustained Drug Delivery
These systems can remain in the stomach for long periods and hence can release the drug over a prolonged period of time. The problem of short gastric residence time encountered with an oral CR formulation hence can be overcome with these systems. These systems have a bulk density of <1 as a result of which they can float on the gastric contents. These systems are relatively large in size and passing from the pyloric opening is prohibited.
Site-Specific Drug Delivery
These systems are particularly advantageous for drugs that are specifically absorbed from stomach or the proximal part of the small intestine, e.g. riboflavin and furosemide. Floating microspheres can greatly improve the pharmacotherapy of the stomach through local drug release, leading to high drug concentrations at the gastric mucosa, thus eradicating Helicobacter pylori from the submucosal tissue of the stomach and making it possible to treat stomach and duodenal ulcers, gastritis and oesophagitis.
Absorption Enhancement
Floating microspheres are especially effective in delivery of sparingly soluble and insoluble drugs. It is known that as the solubility of a drug decreases, the time available for drug dissolution becomes less adequate and thus the transit time becomes a significant factor affecting drug absorption. For weakly basic drugs that are poorly soluble at an alkaline pH, hollow microspheres may avoid chance for solubility to become the rate-limiting step in release by restricting such drugs to the stomach. The positioned gastric release is useful for drugs efficiently absorbed through stomach such as Verapamil hydrochloride. The gastro-retentive floating microspheres will alter beneficially the absorption profile of the active agent, thus enhancing its bioavailability.
As carriers
The floating multiparticulates can be used as carriers for drugs with so-called absorption windows, these substances, for example antiviral, antifungal and antibiotic agents (Sulphonamides, Quinolones, Penicillins, Cephalosporins, Aminoglycosides and Tetracyclines) are taken up only from very specific sites of the GI mucosa. Pharmacokinetic advantages and future potential: As sustained release systems, floating dosage forms offer various potential advantages evident from several recent publications. Drugs that have poor bioavailability because their absorption is restricted to the upper GI tract can be delivered efficiently thereby maximizing their absorption and improving their absolute bioavailabilities. 19, 21
Table 1: List of recently marketed drug formulation utilizing FDDS
|
S. No. |
Dosage form |
Drug |
Polymer |
Method |
Ref. |
|
1.
|
Multiparticulate FDDS |
Zolpidem tartarate |
(Eudragit® NE 30D) |
Gas generation technique |
22 |
|
2. |
Floating microspheres |
Cephalexin |
EthylCellulose (EC) |
Emulsion solvent evaporation |
23 |
|
3. |
Hollow microspheres |
Ranitidine HCl |
Eudragit RLPO |
Solvent evaporation method |
24 |
|
4. |
Floating microparticles |
Metoprolol succinate |
polymethacrylate (Eudragit S100, RSPO, RLPO) |
Non-aqueous emulsion solvent evaporation method |
25 |
|
5. |
Drug-loaded beads |
Pantoprazole |
Alginate, Sterculia gum |
Ionotropic gelation |
26 |
|
6. |
Floating matrix tablets |
Acyclovir |
Hydroxypropyl lulose 4000 |
Gas generation technique 40 |
27 |
|
7. |
Floating microspheres |
Aceclofenac |
Eudragit S 100 (ES) :Eudragit RL 100
|
Emulsion solvent diffusion technique |
28 |
|
8. |
Effervescent floating tablets |
Metformin HCl
|
Methocel K100M and Methocel E50 (4:1) |
Gas generation technique |
29 |
|
9. |
Floating microspheres |
Aceclofenac |
Eudragit RS 100 |
Emulsification solvent evaporation technique |
30 |
|
10. |
Floating tablets |
Aceclofenac |
HPMC E5M and Eudragit RS 100 |
Compression |
31 |
|
11. |
Floating tablets |
Diltiazem HCl |
Xanthan gum as carrier |
Gas generation technique |
32 |
|
12. |
Floating tablets |
Metronidazole |
Methocel K100M CR |
Gas generation technique |
33 |
|
13. |
Self-emulsifying floating pellet |
Tetrahydrocu rcumin |
Glyceryl behenate and sodium starch glycolate |
-- |
34 |
|
14. |
Floating matrix tablets |
Antiretroviral drug |
Hydroxypropyl methylcellulose |
Gas generation technique |
35 |
|
15. |
Effervescent floating tablet |
Diltiazem HCl |
HPMC and xanthan gum |
Gas generation technique |
36 |
|
16. |
Superporous hydrogel composite |
Ranitidine HCl |
sodium carboxy |
- |
37 |
|
17. |
Effervescent floating tablets |
Famotidine |
Carbopol® 71G and Cellactose® 80 |
Gas generation technique |
38 |
|
18. |
Floating tablets |
Verapamil HCl |
Carbopol |
Gas generation technique |
39 |
|
19. |
Floating alginate beads |
Levofloxacin
|
Hemihydrate Methyl cellulose |
Gas generation technique |
40 |
|
20. |
Floating bead formulation |
Nevirapine |
Hydroxypropyl methylcellulose |
Gas formation technique |
41 |
|
21. |
Cinnarizine-loaded EMG beads |
Cinnarizine |
HPMC K4M, HPMC K100M |
Emulsion-gelation method |
42 |
|
22. |
Sustained-release matrices |
Metoprolol succinate
|
Gelucire 43/01 and Gelucire 44/14 |
Melt-solidification technique |
43 |
Reference:
1. Streubel A, Siepmann J, Bodmeier R. Multiple unit gastroretentive drug delivery systems. A new preparation method for low density microparticles. J. Microencapsul. 2003; 20: 329-347.
2. Lim F, Sun AM, Microencapsulated islets as bioartificial endocrine pancreas, science. 1980; 210: 908-910.
3. Dey NS, Majumdar S and Rao MEB. Multiparticulate Drug Delivery Systems for Controlled Release. Trop J Pharm Res. 2008; 7(3):1067-1075.
4. Talukder R, Fissihi R, Gastroretentive Delivery Systems. A Mini review. Drug Dev. and Ind. Pharm. 2004; 30(10): 1019-1028.
5. Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. Physicochemical properties to determine the buoyancy of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur. J. Pharm. Biopharm. 2003; 55: 297-304.
6. Streubel A, Siepmann J, Bodmeier R. Floating microparticles based on low density foam powder. Int. J. Phar. 2002; 241: 279-292.
7. Patil JS, Kamalapur MV, Marapur SC, Kadam DV. Ionotropic gelation and polyelectrolyte complexation. The novel techniques to design hydrogel particulate sustained, modulated drug delivery system a review. Dig. J. of nanomaterials and nanostructures. 2010; 5: 241-248.
8. Manjanna KM. Formulation of oral sustained release aceclofenac sodium microbeads. Int. J. Pharm. Tech. Research. 2009; 1(3): 940-952.
9. Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system. J. Pharm. Sci. 1992; 81: 135-140.
10. Chickering DE, Jacob JS, Matho WE. Reactive Polymers. 1995;(25):189-206
11. Gibaly I. Development and evaluation of novel floating chitosan microcapsules for oral use. Comparison with non floating chitosan microspheres. Int. J. Pharm. 2002; 249: 7- 21.
12. Martin. A, Swarbrick J, Cammarata A. Physical Pharmacy, III Ed, Varghese Publishing Company, Bombay. 1991; 492- 520.
13. Umamaheshwari RB, Jain S, Bhadra D, Jain NK. Floating for the treatment of Helicobacter pylori. Int. J. Pharm. 2003; 55(12): 1607-1613.
14. Jain NK. Progress in Controlled and Novel Drug Delivery Systems, 1Ed. CBS Publishers and Distributors, New Delhi, Bangalore, 2004; 84-85.
15. Gholap SB, Banarjee SK, Gaikwad DD, Jadhav SL, Thorat RM. Hollow microsphere. A review. Int J Pharmacy and Pharm Sci. March-April 2010. 1(1), 210-220.
16. Whitehead L, Fell JT, Collett JH, Sharma HL, Smith A. In-vivo study demonstrating prolonged gastric retention. J. Con. Rel. 1998; 55: 312.
17. Menon A, Ritschel WA. Development and evaluation of a monolithic floating dosage form for furosemide. J. Pharm. Sci. 1994; 83: 239-245.
18. Mayavanshi AV and Gajjar SS. Floating Drug Delivery Systems to Increase Gastric Retention of Drugs: A eview. 2008: 345-348.
19. Kavitha K, Yadav SK and Tamizh MT. The Need of Floating Drug Delivery System: A Review. RJBPS. 2010; (2): 396-405.
20. Vyas SP, Khar R.K. Controlled Drug Delivery, concepts and advances. Vallabh Prakashan. 2002. 196-217.
21. Somwanshi SB, Dolas RT, Nikam VK, Gaware VM, Kotade KB, Dhamak KB and Khadse AN. Floating Multiparticulate Oral Sustained Release Drug Delivery System. J.Chem.Pharm Res. 2011; 3(1): 536-547.
22. Amrutkar PP, Chaudhari PD, Patil SB. Design and in vitro evaluation of multiparticulate floating drug delivery system of zolpidem tartarate. Colloids and Surfaces B: Biointerfaces. 2012; 89(1):182-7.
23. Vasava K, Rajesh KS, Jha LL. Formulation and evaluation of floating microspheres of Cephalexin. International Journal of Pharmaceutical Sciences Review and Research. 2011; 11(2):69-75.
24. Singh V, Chaudhary AK. Preparation of Eudragit E100 microspheres by modified solvent evaporation method. Acta poloniae pharmaceutica. 2011; 68(6):975-80.
25. Nadigoti J, Dharani S, Shayeda, Yamsani MR. Formulation and evaluation of floating microparticles of metoprolol succinate. Asian Journal of Pharmaceutical and Clinical Research. 2011; 4(SUPPL. 1):132-5.
26. Singh B, Chauhan D. Barium ions crosslinked alginate and sterculia gum-based gastroretentive floating drug delivery system for use in peptic ulcers. International Journal of Polymeric Materials. 2011; 60(9):684-705.
27. El Gamal SS, Naggar VF, Allam AN. Optimization of acyclovir oral tablets based on gastroretention technology: Factorial design analysis and physicochemical characterization studies. Drug Development and Industrial Pharmacy. 2011; 37(7):855-67.
28. Tamizharasi S, Sivakumar T, Chandra RJ. Formulation and evaluation of floating drug delivery system of aceclofenac. International Journal of Drug Development and Research. 2011; 3(3):242-51.
29. Nasa P, Mahant S. Floating drug delivery system using methocel k100m and e50: Formulation and characterization. Acta Pharmaceutica Sciencia. 2011; 53(1):57-65.
30. Kancharla K, Basavaraj BV, Bharath S, Deveswaran R, Madhavan V. Formulation and evaluation of intragastric floating multiparticulate system of Aceclofenac. Der Pharmacia Lettre. 2011; 3(2):238-45.
31. Reddy AB, Samyuktha Rani B, Eswar Tony D, Sivanaga Raja D, Sindhura L, Sarath Kumar N. Aceclofenac floating tablets - A promising sustained release dosage form. International Journal of Drug Development and Research. 2011; 3(2):290-300.
32. Subhash Chandra Bose P, Srikanth Reddy P, Ravi V, Sarita D, Pramod Kumar TM. Formulation and evaluation of sustained release floating tablets of diltiazem HCl using xanthan gum. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2011; 2(2):319-28.
33. Juárez-Soberanez D, Villafuerte-Robles L. Gelucire 39/01 as excipient for gastroretentive metronidazole sustained delivery. International Journal of Pharmacy and Pharmaceutical Sciences. 2011; 3(SUPPL. 2):86-91.
34. Setthacheewakul S, Kedjinda W, Maneenuan D, Wiwattanapatapee R. Controlled release of oral tetrahydrocurcumin from a novel Selfemulsifying Floating Drug Delivery System (SEFDDS). AAPS PharmSciTech. 2011; 12(1):152-64.
35. Verma S, Narang N. Development and in vitro evaluation of floating matrix tablets of anti retroviral drug. International Journal of Pharmacy and Pharmaceutical Sciences. 2011; 3(1):208- 11.
36. Amrutkar PP, Chaudhari PD, Patil SB. Design and in vitro evaluation of multiparticulate floating drug delivery system of zolpidemn tartarate. Colloids and Surfaces B: Biointerfaces. 2012; 89(1):182-7.
37. Siddiqui AI, Bakde BV, Tappar KK. Floating strategy for low absorption window diltiazem hydrochloride. International Journal of Pharmacy and Technology. 2011; 3(1):1893- 903.
38. Chavda H, Patel C. Preparation and in vitro evaluation of a stomach specific drug delivery system based on superporous hydrogel composite. Indian Journal of Pharmaceutical Sciences. 2011; 73(1):30-7.
39. Nalawade P, Somvanshi A, Dand N, Kadam V, Hirlekar R. Formulation and optimization of directly compressible floating tablets of famotidine using 23 factorial design. Latin American Journal of Pharmacy. 2010; 29(6):991-9.
40. Krishnan V, Sasikumar S, Dass C FP, Vijayaraghavan R. Effect of pore forming agents on the physical characteristics and release kinetics of levofloxacin hemihydrate from floating alginate drug delivery system - An in vitro study. Trends in Biomaterials and Artificial Organs. 2010; 24(3):139-45.
41. Krishnan V, Sasikumar S, Dass C FP, Vijayaraghavan R. Effect of pore forming agents kinetics of levofloxacin hemihydrate from floating alginate drug delivery system - An in vitro study. Trends in Biomaterials and Artificial Organs. 2010; 24(3):139-45.
42. Vedha Hari BN, Brahma Reddy A, Samyuktha Rani B. Floating drug delivery of nevirapine as a gastroretentive system. Journal of Young Pharmacists. 2010; 2(4): 350-5.
43. Prajapati BG, Patel RP, Vaghasia KL. Controlled release of cinnarizine using gasto-retentive emugel beads of calcium alginate. Drug Delivery Technology. 2010;10(7):46
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









