 About Authors:
About Authors:
Patel Pinkesh*1, Patel Chirag J1, Prof. Satyanand Tyagi2, Umesh Kumar1, Patel Jaimin1, Chaudhari Bharat1
1Department of Pharmaceutics, Maharishi Arvind Institute of Pharmacy, Mansarovar, Jaipur, Rajasthan, India-302020.
2President & Founder, Tyagi Pharmacy Association (TPA) & Scientific Writer (Pharmacy), Chattarpur, New Delhi, India-110074.
*pinkesh.patan@yahoo.com, +91-8866436904
ABSTRACT:
In the last decade, interest in developing a combination of two or more Active Pharmaceutical Ingredients (API) in a single dosage form (bilayer tablet) has increased in the pharmaceutical industry. Pharmacokinetic profile relies on the fact that the fast release layer provide the loading dose of drug and the sustained release of drug maintain the drug concentration within therapeutic window for longer period of time. Bilayer tablets offer definite advantages over conventional release formulation of the same drug. Now a day, several pharmaceutical companies are developing bilayer tablet for co-administration of drugs to improve the therapeutic efficacy as well as to reduce the chances of drug-drug interaction.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1566
INTRODUCTION
Oral ingestion has long been the most convenient and commonly employed route of drug delivery due to its ease of administration. It is well known that modified release dosage forms may offer one or more advantages over immediate release formulations of the same drug. There are many ways to design modified release dosage forms for oral administration; from film coated pellets, tablets or capsules to more sophisticated and complicated delivery systems such as osmotically driven systems, systems controlled by ion exchange mechanism, systems using three dimensional printing technology and systems using electrostatic deposition technology. The design of modified release drug product is usually intended to optimize a therapeutic regimen by providing slow and continuous delivery of drug over the entire dosing interval whilst also providing greater patient compliance and convenience [1, 2]. The most common controlled delivery system has been the matrix type such as tablets and granules where the drug is uniformly dissolved or dispersed throughout the polymer, because of its effectiveness, low cost, ease of manufacturing and prolonged delivery time period [3].
Usually conventional dosage form produce wide ranging fluctuation in drug concentration in the blood stream and tissues with consequent undesirable toxicity and poor efficiency. This factor such as repetitive dosing and unpredictable absorption led to the concept of controlled drug delivery systems. The goal in designing sustained or controlled delivery systems is to reduce the frequency of the dosing or to increase effectiveness of the drug by localization at the site of action, reducing the dose required or providing uniform drug delivery. The primary objective of sustained release drug delivery is to ensure safety and to improve efficacy of drugs as well as patient compliance [4].
Bi-layer tablet is suitable for sequential release of two drugs in combination, separate two incompatible substances and also for sustained release tablet in which one layer is immediate release as initial dose and second layer is maintenance dose. There is various application of the bi-layer tablet it consist of monolithic partially coated or multilayered matrices. In the case of bi-layered tablets drug release can be rendered almost unidirectional if the drug can be incorporated in the upper non-adhesive layer its delivery occurs into the whole oral cavity [5].
ADVANTAGES OF BILAYER TABLET
The advantages of the bilayer tablet over the other conventional preparations of oral solid dosage forms include:
1. When the two different layers of the tablet content two different drugs, then the tablet can be easily used in combination therapy.
2. Frequency of the dose administration is reduced which ultimately improve the patient compliance.
3. This formulation can be use to deliver separate two incompatible substance.
4. In case of drugs having a low half life, each of the two layers of the tablet respectively content a loading dose and maintenance dose of the same and thus increase the bioavailability of the drug.
5. In case of a conventional dosage form due to fluctuation of the dose interval the plasma drug concentration may differ (under medication or over medication), but in this dosage form the plasma drug concentration is always constant, which ultimately provide a more effective action of the drug.
6. Greatest chemical and microbial stability over all oral dosage form.
7. Better control of drug absorption can be attained, since the high blood level peaks that may be observed after administration of a dose of high availability drug can be reduced by formulation in an extended action form. The safety margin of high potency drugs can be increased and the local and systemic adverse effects can be reduced in sensitive patients.
8. Suitable for large scale production [6-8].
[adsense:468x15:2204050025]
LIMITATIONS OF BILAYER TABLET
From the above mentioned advantage of bilayer tablets it is quite clear that in pharmaceutical industry it is a great revolution, but there are certain limitations in the formulation and use of bilayer tablets, such as:
1. Drugs with poor wetting, slow dissolution properties, optimum absorption high in GIT may be difficult to formulate or manufacture as a tablet that will still provide adequate or full drug bioavailability.
2. One of the major challenges in bilayer formulation is lack of sufficient bonding and adhesion at the interface between the adjacent compacted layers which is often the result of an interfacial crack and layer separation.
3. Difficult to swallow in case of children and unconscious patients.
4. If the compacted layers are too soft or too hard, they will not bind securely with each other which can lead to compromised mechanical integrity and also the separation of the layers.
5. Other challenges during development include establishing the order of layer sequence, layer weight ratio, elastic mismatch of the adjacent layers, first layer tamping force, and cross contamination between layers.
6. The adjacent layers of a bilayer tablet are bonded together by mechanical means, so the factors influences the stress state is very important. The mechanical properties of each layer and the tablet, and compression parameters along with specialized techniques and compression condition plays a very important role for the same.
7. Administration of sustained release bilayer tablet does not permit the prompt termination of therapy.
8. Bitter testing drugs, drugs with an objectionable odour or drugs that are sensitive to oxygen may require encapsulation or coating.
9. The physician has a less flexibility on adjusting the dose regimens [6, 8, 9].
GENERAL PROPERTIES OF BI-LAYER TABLET DOSAGE FORMS
1. A bi-layer tablet should have sufficient strength to withstand mechanical shock during its production packaging, shipping and dispensing.
2. A bi-layer tablet should have elegant product identity while free of defects like chips, cracks, discoloration, and contamination.
3. A bi-layer tablet should have the chemical and physical stability to maintain its physical attributes over time. The bi-layer tablet must be able to release the medicinal agents in a predictable and reproducible manner [6, 8].
GMP REQUIREMENTS FOR BILAYER TABLET
To produce a quality bi-layer tablet, in a validated and GMP-way, it is very important to follow the following criteria for the selection of bilayer press. These requirements seem obvious but are not so easily accomplish. The press should be capable of:
1. Preventing capping and separation of the two individual layers that constitute the bi-layer tablet;
2. Preventing cross-contamination between the two layers;
3. Providing sufficient tablet hardness;
4. Accurate and individual weight control of the two layers;
5. Producing a clear visual separation between the two layers;
6. Manufacturing products of high yield [6, 9].
VARIOUS APPROACHES USED IN THE BILAYER TABLET
A. Floating Drug Delivery System
These are designed to have a low density and thus float on gastric contents after administration until the system either disintegrates or the device absorbs fluid to the point where its density is such that it loses buoyancy and can pass more easily from the stomach with a wave of motility responsible for gastric emptying. The bilayer tablet is designed in such a manner that, one layer gives the immediate dosing of the drug which gives faster onset of action while other layer is designed as a floating layer which floats in the stomach (GI-fluid).
Disadvantages: It may not have the controlled loss of density alternatively required for it to eventually exit from the stomach. Floating tablets are not applicable to higher dose levels of highly water soluble drugs where large amounts of polymer are needed to retard drug release, as in case of water soluble drugs. The performance of floating formulation may also be posture dependant. A patient sitting upright may ensure prolonged gastric residence of a buoyant dosage form, whereas a supine patient might allow ready presentation of the floating dosage form to the pylorus and thus allow rapid exit of the dosage form from the stomach. Hence, floating dosage forms might be expected to only have limited applications.
B. Polymeric Bioadhesive System
These are designed to imbide fluid following administration such that the outer layer becomes a viscous, tacky material that adheres to the gastric mucosa/mucus layer. This should encourage gastric retention until the adhesive forces are weakened. These are prepared as one layer with immediate dosing and other layer with bioadhesive property.
Disadvantages: The success seen in animal models with such system has not been translated to human subjects due to differences in mucous amounts, consistency between animals and humans.
The system adheres to mucous not mucosa. The mucous layer in humans would appear to slough off readily, carrying any dosage form with it. Therefore, bioadhesive dosage form would not appear to offer a solution for extended delivery of drug over a period of more than a few hours.
C. Swelling System
These are designed to be sufficiently small on administration so as not to make ingestion of the dosage form difficult (e.g., less than approximately 23 mm long and less than 11 mm wide for an oval or capsule –shaped tablet whereas 10- 12mm in diameter for round tablets). On ingestion they rapidly swell or disintegrate or unfold to a size that precludes passage through the pylorus until after drug release has progressed to a required degree. Gradual erosion of the system or its breakdown into smaller particles enables it to leave stomach. The simple bilayer tablet may contain an immediate release layer with the other layer as extended release or conventional release [10].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
INFLUENCE OF PROCESS AND FORMULATION PARAMETERS
As the initial dose layer do not affect the intermediate slow release or the second rapid phase or constant phase release, this layer is not necessary to be considered in the formulation process. Multi-layered tablet consisting of a core and one or more barrier layers should be taken into account while determining the parameters involved in the processing. The following factors should be considered for the process and formulation [11].
1. Parameters dealing with the layer consisted of therapeutically active substances
During granulation of therapeutically active substances some basic factors are to be considered which includes percentage of the liquid used in granulation, time required for massing step, temperature of the outlet air during the drying step and milling screen apertures as well as the interaction between the amount of granulation liquid and the outlet temperature. While the impact of these factors on the final products has to be considered and the responses can be classified into four categories: (i) granules properties (e.g., flowability, bulk density, ability to settle, particle size distribution), (ii) extensometric responses (e.g. cohesion index, lubrication index, ejection strength, plasticity, elasticity), (iii) physical characteristics of tablet (e.g. thickness, weight variation, hardness, friability) and (iv) analytical results (e.g. content uniformity, in vitro profile) [12].
2. Compression process
The critical parameters in the compression process are turntable speed and compression forces corresponding to first, second and main layers. The tablet crushing strength response improves when the turret compression speed on the main compression force is increased. But these parameters (within a particular range) do not influence the content uniformity and the release performances in multi-layered, press coated and, bimodal delivery systems [13, 14]. But in the case of press coated tablet intended for distant destination (e.g. colon targeting) the release rate and lag time are dependent on the compression force. The release rate of drug decreases and the lag time increases with increasing compression force till a critical point. After this point increasing compression force does not provide further reduction in porosity. There is necessity of increasing the lag time more than 10 h in the gastric fluid under some physiological conditions [15] and also there is need for suppression of release in the intestinal fluid for more than 3 h in order to obtain colon targeting. To achieve these certain additives, which have poor wettability, are added to the outer shell polymer to prevent the penetration of dissolution medium into the pores in the outer shell. For example, magnesium stearate or calcium stearate were added to the hydroxyl propyl methyl cellulose acetate succinate (HPMCAS) polymer to increase the lag time [16]. Eiji Fukui et al. [16] reported that the drug release in gastric fluid was completely suppressed until 15 h if tablets containing magnesium stearate irrespective of compression force and for tablets containing calcium sterate, it was necessary to increase the compression force to more than the range applied, to suppress until 12 h. In the intestinal fluid the lag time was not prolonged to more than 2 h by addition of magnesium sterate. In contrast lag time could be prolonged by calcium stearte as long as 9 h by increasing the compression force. The above results suggested that press coated tablets intended for colon targeting mainly depends on compression force when poor wettable additives are used.
3. Hardness of compressed tablet
The resistance of tablets to shipping or breakage under conditions of storage, transportation and handling before usage depends on its hardness. The hardness of tablet of each formulation was measured by Monsanto hardness tester. The hardness was measured in kg/cm2.
Hardness of tablet is expressed in terms of tensile strength. The tensile strength of the tablet is calculated by the formula, according to Fell and Newton [18]:
σ=2P/πDt
Where r = tensile strength (kg/cm2), D= tablet diameter (cm), t = tablet thickness (cm), P= force applied to fracture (kg). The porosity of the tablets decreased by the rise of tensile strength which is ultimately depends on the compression load. Since the compression force (particular range) does not affects the release rate, therefore, hardness of the tablet (generally in layered construction) has less significance in the formulation [14].
4. Polymer concentration in core
Polymer is one of the most important factors that influence the release of drug from the tablets. With the increase of the polymer concentration usually the dissolution rate of the tablet is decreased. This parameter does not affect the drug release in layered tablets as considerably in the bimodal tablet because the solubility of certain polymers depends on the pH of the surrounding medium. For example, the effect of decreasing HPMCAS-MF amounts in the inner layer of bimodal delivery system is not significant in pH 1.2 but in pH 7.4 drug release increases with decrease in the amount of polymers. At high pH values a less dense polymer network dissolves more rapidly than a tight structure, leading to increased drug release rate. At low pH HPMCAS-MF is not soluble, thus there is no effect on the breakdown of the polymer network. Therefore, concentration of pH sensitive retard polymers in the core should be controlled more closely [12].
5. Filler
Filler used in the core of the tablet, has a great influence on the drug release rate because of its solubility. On contact with the release medium, the filler diffuses out from the device and thereby affect the drug release rate by increasing the porosity of polymers. Depending upon the amount of the filler the amount of the polymer is adjusted to keep the tablet weight constant. Example of such filler is lactose [12, 14].
VARIOUS TECHNIQUES FOR BILAYER TABLET
1. Osmotic-controlled release oral delivery system
In this technology the system is consist of mainly two or three layer among which one or more layer are of the drug and other layers are consist of push layer (Fig. 1). The drug layer mainly consists of poorly soluble drug along with diluents, low molecular weight polymer, suspending agent and osmotic agent. The push layer is constructed of a higher molecular weight osmopolymer and an osmagent. A semi permeable membrane surrounds the tablet core. In this technology the medication is sandwiched with an osmotic agent that swells when it takes up water. The sandwich is then coated with a semi permeable membrane .Then a laser is used to drill a tiny hole through the membrane. In the stomach, water passes through the membrane into the pill, causing the osmotic material to swell, which pushes the drug out of the hole. This delivers the drug to the body at a constant rate instead of all at once, as happens when a traditional pill dissolves. Products manufactured using this technology are Glucotrol Xland procardia XL both of which are composed of a bilayer tablet core and Concerta iscompose of a trilayer tablet core [10].
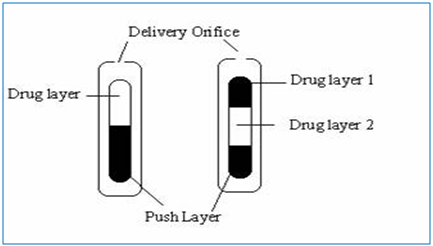
Figure 1: Preparation of bilayer and trilayer tablet [10].
2. Geomatrix Technologies
Geomatrix system is a multilayer tablet with a matrix core containing the active ingredient and one or more modulating layers (barriers) applied to the core during the tableting process. The function of these barriers is to delay the interaction of the core with the dissolution medium. Eight Geomatrix technologies are designed to meet a wide range of therapeutic objectives: Zero-order release provides a constant rate of drug release over a defined period of time; binary release is used to provide the controlled release of two different drugs in a single tablet; quick–slow release provides a quick burst of drug release followed by a constant rate of release over a defined period of time; slow–quick release provides an initial constant rate of release followed by a quick burst of drug release at a predetermined time; positioned release delivers the drug to a predetermined position in the digestive system before it begins to release the active drug compounds; accelerated release provides a constantly accelerating rate of drug release; delayed release provides a predetermined time lag before it begins releasing drug molecules; multiple pulse provides an initial quick burst of drug release followed by a predetermined period of no release. Some of the drugs that are marketed based on this technology are diltiazem hydrochloride, nifedipine, and diclofenac sodium [18].
3. Elan drug technology (DUREDUS technology)
DUREDAS (Fig. 2, 3) or Dual Release Drug Absorption System (Elan Corporation) utilizes bilayer-tabletting technology, which has been specifically developed to provide two different release rates or dual release of a drug from a single dosage form. The tablets are prepared by two separate direct-compression steps that combine an immediate-release granulate (for rapid onset of action) and a controlled-release hydrophilic matrix complex within one tablet. The immediate release layer, release the drug immediately after going into the GIT (stomach or intestine) in a diffusion and dissolution manner and the controlled-release matrix remains intact and slowly absorbs fluid from the GI tract, which causes the matrix to expand and transforms the hydrophilic polymers into a porous, viscous gel that serves as a barrier between the drug and the surrounding fluid. As the gel continues to expand, fluid penetrates further into the dosage form, dissolving the drug and allowing the resulting solution to diffuse out in a controlled manner. A further extension of the Duredus technology is the production of controlled-release combination dosage forms whereby two different drugs are incorporated into the different layers, and the drug release of each is controlled to maximize therapeutic effect of the combination. Again both immediate release and controlled release combinations of the two drugs are feasible. The DUREDAS™ technology was initially employed in the development of a number of over the counter controlled release analgesics[6-9, 19].
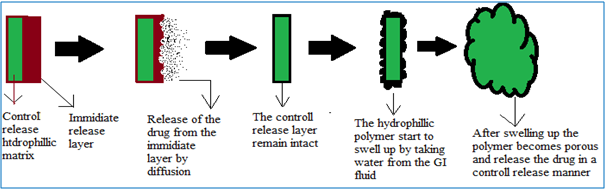
Figure 2: DUREDAS technology consists of control release and immediate release layer [19].
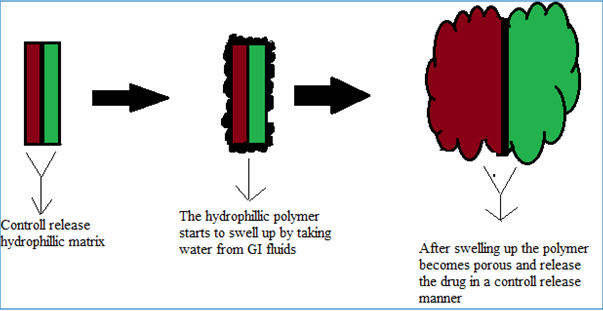
Figure 3: DUREDAS technology consist of two control release layers [19].
VARIOUS COMBINATIONS OF BILAYER TABLETS
Table 1 indicates the different formulations of bilayer tablet containing combination of two drug and their specific uses. Table 2 indicates the different bilayer tablet formulation available in the market.
Table 1: Bilayer tablets containing two drugs in an individual layer.
|
Drug: 1 |
Drug: 2 |
Purpose |
Ref. |
|
Tramadol
|
Acetaminophen |
Prolonged release up to 12 h and improve patient compliance |
20 |
|
Metformin hydrochloride |
Glimepiride |
Improve oral therapeutic efficacy with optimal control of plasma drug level |
21 |
|
Metformin hydrochloride |
Pioglitazone |
Reduce frequency of administration and improve patient compliance |
22 |
|
Paracetamol |
Diclofenac sodium |
Reduce dose frequency and decrease incidence of GI side effects |
23 |
|
Atorvastatin calcium |
Nicotinic acid |
Develop potential dosage form |
24 |
|
Metoclopramide hydrochloride |
Ibuprofen |
Effective treatment of migraine and avoid chemical incompatibility between drugs |
5 |
|
Metoprolol succinate
|
Amlodipine besylate |
Lower doses of drug to reduce patient blood pressure, minimize dose dependent side effects and adverse reactions |
25 |
|
Diltiazem hydrochloride |
Lovastatin |
Improve patient compliance and better disease management |
26 |
|
Salbutamol
|
Theophylline |
Enhance patient compliance and prolong bronchodilation |
27 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 2: Commercially marketed bilayer tablets [19]
|
Product Name |
Chemical Name |
Developer |
|
Glycomet®-GP2Forte |
Metformin hydrochloride, Glimepiride |
USV Limited |
|
ALPRAX PLUS |
Sertraline, Alprazolam |
Torrent Pharmaceutcals Ltd. |
|
DIAMICRON®XRMEX500 |
Gliclazide, Metformin hydrochloride |
Sedia® Pharmaceuticals (India) Pvt. Ltd. |
|
Newcold Plus |
Levocetrizine hydrochloride, Phenylpropanolamine, Paracetamol |
Piramol Healthcare Ltd. |
|
TRIOMUNE 30 |
Nevirapine, Lamivudine, Stavudine |
Cipla Ltd. |
|
DIUCONTIN-K®20/250 |
Furosemide, Potassium chloride |
T.C. Health Care Pvt. Ltd. |
|
Tribet-1 |
Glimepiride, Pioglitazone hydrochloride, Metformin hydrochloride |
Abbott Healthcare Pvt. Ltd. |
|
PIOKIND®-M15 |
Pioglitazone, metformine hydrochloride |
Psychotropics India Ltd. |
|
Revelol®-Am 25/5 |
Metoprolol succinate, Amlodipine besilate |
Ipca Laboratories Ltd. |
EVALUATION OF SUSTAIN RELEASE BILAYER TABLET [6, 19, 26]
1. Tablet Thickness and Size
Thickness and diameter of tablets were important foruniformity of tablet size. Thickness and diameter was measuredusing venire caliper.
2. Tablet Hardness
The resistance of tablets to shipping or breakage underconditions of storage, transportation and handling before usagedepends on its hardness. The hardness of tablet of each formulationwas measured by Monsanto hardness tester. The hardness wasmeasured in kg/cm2.
3. Friability
Friability is the measure of tablet strength. Electrolab EF- 2 friabilator (USP) was used for testing the friability using the following procedure. Twenty tablets were weighed accurately and placed in the tumbling apparatus that revolves at 25 rpm dropping the tablets through a distance of six inches with each revolution. After 4 min, the tablets were weighed and the percentage loss in tablet weight was determined.
% loss = [(Initial wt. of tablets – Final wt. of tablets)/ Initial wt. of tablets] ×100
4. Uniformity of weight
Twenty tablets were selected at random and the average weight was calculated. Weight Variation was calculated and was compared with I. P. standards.
5. Dissolution Studies
Bilayer tablets were subjected to in vitro drug release studies in simulated gastric and intestinal fluids to assess their ability in providing the desired controlled drug delivery. Drug release studies were carried out using USP dissolution test apparatus I at 100 rpm, 37±0.5°C, and pH 1.2 buffer (900 ml) (i.e. 0.1 N HCl) for 2 hours, since the average gastric emptying time is about 2 hours. The dissolution medium was replaced with pH 6.8 phosphate buffer (900ml) and experiment continued for another 10 hours. At different time intervals, 5ml of the samples were withdrawn and replaced with 5ml of drug-free dissolution medium. The samples withdrawn were analyzed by UV spectrophotometer using multi component mode of analysis.
CONCLUSION AND DISCUSSION
Bilayer tablet is suitable for sequential release of two drugs in combination, separate two incompatible substances and also for sustained release tablet in which one Layer is immediate release as initial dose and second layer is maintenance dose. Compression Force-controlled presses are clearly limited when a quality bi-layer tablet needs to be produced in conjunction with accurate weight control of both layers. Low pre-compression forces are necessary to secure interlayer bonding. But at low forces, the compression force control system is not sufficiently sensitive and therefore lacks in accuracy. The use of higher compression forces may rapidly result in separation and hardness problems when compressing bi-layer tablets. Such problems become even more apparent when the tableting speed is high or increased.
REFERENCES:
1. Wilding IR, Coupe AJ, Davis SS. The role of gamma scintigraphy in oral drug delivery. Adv. Drug Deliv. Rev.1991; 7:87–117.
2. Chien YW. Fundamentals of controlled-release of drug administration in: J. Swarbrick (Ed.), Novel Drug Delivery System Marcel Dekker, New York, 1982, pp. 465–574.
3. Lee L. Diffusion-controlled matrix systems, in: A. Kydonieus (Ed.), Treatise on Controlled Drug Delivery, Marcel Dekker, New York, 1992, pp. 155– 198.
4. Kumar KK, Mahesh M, Sasikanth K. Design, development and characterization of sustained release of Metformin hydrochloride and Gliclazide bilayered tablets by wet granulation method. Int J Biopharm 2010; 1(2):67-71.
5. Shiyani B, Gattani S, Surana S. Formulation and evaluation of bi-layer tablet of Metoclopramide hydrochloride and Ibuprofen. AAPS Pharm Sci Tech 2008 ; 9(3):818-27.
6. Deshpande RD, Gowda DV, Mahammed N, Deepak N. Maramwar. Bi-layer tablets- An emerging trend: a review. IJPSR 2011; 2(10): 2534-2544.
7. Panchal HA, Tiwari AK. Novel Approach of Bilayer tablet Technology: An Review. Journal of Pharmaceutical Science and Technology 2012; 4(4): 892–904.
8. Divya A, Kavitha K, Kumar MR, Dakshayani S, Jagadeesh SSD. Bilayer tablet technology: An overview. Journal of Applied Pharmaceutical Science 2011; 01(08): 43-47.
9. Patel M, Ganesh NS, Kavitha, Tamizh M. Challenges in the formulation of bilayered tablets: A review. IJPRD 2010; 2(10): 30–42.
10. Panchal HA, Tiwari AK. Novel Approach of Bilayer tablet Technology: An Review. Journal of Pharmaceutical Science and Technology 2012; 4(4), 892–904
11. Vinayagamkannan, Ragupathikandarapu, Garg S. Optimization techniquies for the design and development of novel drug delivery systems- part I. Pharm. Technol 2003; 27(2): 74–90.
12. Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986; 27: 886–92.
13. Ahmed SI, Mangamoori LN, Rao YM. Formulation and characterization of matrix and triple-layer matrix tablets for oral controlled drug delivery. International Journal of Pharmacy and Pharmaceutical Sciences 2010; 2(3): 137–43.
14. Rane AB, Gattani SG, Kadam VD, Tekade AR. Formulation and evaluation of press coated tablets for pulsatile drug delivery using hydrophilic and hydrophobic polymers. Chem Pharm Bull 2009; 57(11): 1213–7.
15. Fell JT, Newton JM. Determination of tablets strength by the diametral-compression test. J. Pharm. Sci 1970; 59: 688–91.
16. Fukui E, Miyamura N, Kobayashi M. An in vitro investigation of the suitability of press-coated tablets with hydroxypropylmethylcellulose acetate succinate (HPMCAS) and hydrophobic additives in the outer shell for colon targeting. J. Control. Release 2001; 70: 97–107.
17. Cremer K, Asmussen B. Novel Controlled-release tablet with erodible layers. Proc. Int. Control. Release Bioact. Mater 1995; 22: 732–3.
18. Verma RK, Garg S. Current Status of Drug Delivery Technologies and Future Directions. Pharmaceutical Technology On-Line 2001; 25(2): 1–14.
19. Sanjay D, Sanhati SR, Amitava G, Bhaskar M. Bilayer Table Technology: A Suitable Approach for Bimodal Release and Co-administration of Drug(s) Through Oral Route. Available on: www.apjtb.com/press/2012/B189.doc.
20. Naeem MA, Mahmood A, Khan SA, Shahiq Z. Development and evaluation of controlled release bilayer tablets containing microencapsulated tramadol and acetaminophen. Trop J Pharm Res 2010; 9(4): 347-54.
21. Pattanayak DP, Dinda SC. Bilayer tablet formulation of metformin HCl and glimepiride: A novel approach to improve therapeutic efficacy. Int J Drug Discovery Herb Res 2011; 1(1): 1-4.
22. Ramesh DS, Guruvaiah, Harani A. Formulation and evaluation of bilayer sustained release matrix tablets of metformin Hcl and pioglitazone. Amer-Euras J Sci Res 2010; 5(3): 176-82.
23. Gohel MC, Parikh RK, Nagori SA, Jethwa BA. Fabrication and evaluation of bilayer tablet containing conventional paracetamol and modified release diclofenac sodium. Ind J Pharm Sci 2010; 72(2): 191-6.
24. Nirmal J, Sasivam S, Peddanna C, Muralidharan S, Kumar SG, Nagarajan M. Formulation and evaluation of bilayer tablets of atorvastatin calcium and nicotinic acid. Chem Pharm Bull (Tokyo) 2008; 56(10): 1455-58.
25. Atram SC, Udavant YK, Salunke RJ, Neb GB, Shahi SR, Gulecha BS, Padalkar AN. Formulation and evaluation of bilayer tablet containing metoprolol succinate and amlodipine besylate as a model drug for anti hypertensive therapy. J Pharm Res 2009; 2(8): 1335-47.
26. Kulkarni AS, Manish S. Design and floating bilayer tablets of diltiazen HCl and lovastatin. PDA J Pharm Sci Technol 2008; 62(5): 344-52.
27. Nagaraju R, Kaza R. Formulation and evaluation of bilayer sustained release tablet of salbutamol and theophylline. Int J Pharm Sci Nanotechno 2009; 2(3): 638-46.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









