 About Authors:
About Authors:
Kotta Kranthi kumar1, V Bhargava Reddy2
Sku college of pharmaceutical sciences S.K.University Anantapur
*kranthikumarkotta@gmail.com
ABSTRACT
In the nature so many polymers are available. With this natural polymers will not shows any interactions with the api. In the drug delivery systems the polymers are plays an important role for the delivery of the drugs. By using the polymers we will deliver the drugs in the targeting points. Now a day this polymers science is the emerging field for the development and invention of new polymers. In the coming years polymers will plays a vital role in dug delivery.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1585
INTRODUCTION:
Polymers are natural or synthetic high molecular weight macromolecules made up of repeating monomer units. For the formulation of polymers into drug delivery systems, a thorough understanding of their structure and properties is required. For example, polycarbophil (polyacrylic acid cross-lined with divinyl glycol) was found to be less swollen if the carboxylic acid groups in the molecule are unionized and where as in case there is a high concentration of ionized groups, there is a more influx of water and the particles are highly swollen.
Polymers in drug delivery:
Classification:
Polymers are classified in several ways. For the use in pharmaceutical purposes, they are simple classified into natural and synthetic polymers. As biodegradability plays a major role (important factor) in drug delivery systems, they are further classified into two subgroups as biodegradable and non-biodegradable.
POLYMERS AS PHARMACEUTICAL EXCIPIENTS:
For many years, in pharmaceutical products pharmacists have been employing polymers in every aspects of their work. Some examples of such polymers are polyethylene and polyolefin bottles, polystyrene vials, rubbers closures, rubber and plastic tubing for injection sets and polyvinylchloride flexible bags to hold blood and intravenous solutions. At first in the initial stages, the use of polymers was restricted for only packing rather than drug delivery. The amalgamation of polymer science and pharmaceutical science led to the introduction of polymers in the design and development of drug delivery systems (DDS).
The main intension of these polymeric drug delivery systems was to achieve controlled or sustained drug delivery. Hydro gels, liposomes, bioadhesives, nanoparticles and tissue engineering are some of the most applications of polymers.
Many other polymers which are used as excipients are microcrystalline cellulose (MCC), sodium carboxymethyl cellulose (NaCMC), hyddroxypropulcellulose (HPC), polyethylene glycol (PEG) and povidone are used to coat tablets. Cellulose acetate phthalate, hydroxymethylcellulose phthalate and copolymers of methacrylic acid and its esters, Eudragit are also used for enteric coating of tablets. Targeting of drugs to the colon following oral administration has been done by using biodegradable polysaccharides. Cross-linked guar gum was used as a vehicle in delivery systems for localized delivery to the distal portions of the bowel as its having reduced swelling properties due to the cross-linking.
Polymers like acacia, gelatin and sodium alginate have been used as binders in tableting granulations. HPMC and HPC in hydrated form also act as binders that swell when hydrated by the gastric media and delay absorption. Starch, carboxymethyl starch (CMS) and cross-linked povidone are used as disintegrants. Polyethylene glycol is widely used as a plasticizer to improve elasticity in the preparation of enteric coated tablets. A number of synthetic and natural polymers like Xanthan gum are used to thicken suspensions and opthalmic solutions or also as colloids to stabilize and form water soluble ointment bases.
[adsense:468x15:2204050025]
II) BIODEGRADABLE POLYMERS :
DEFINITION :
A biodegradable polymer is a polymer in which the degradation results from the action of naturally occurring microorganisms such as bacteria, algae or fungi.
Polymers were first developed in the search of biodegradable structures and their applications were found to be useful and successful for long term drug delivery. Biodegradable polymers are highly desirable in their conditions as they degrade in the body to biologically inert and compatible molecules. By incorporating drug into the biodegradable polymers, the dosage forms releases the drug for a long period of time and can be prepared in variety of shapes and sizes. There is no need of any surgical procedures after the completion of the dosing regimen, as the remaining polymer form will be degraded and cleared by the body.
Many different chemistries for biodegradable polymers have been proposed. One of the most common and successful polymers are the polyesters that were first investigated as biodegradable sutures. These polymers include poly (glycolide), poly (D, L-Lactide), and their related copolymers poly (D, L-lactide – co-glycolide). Many commercial products based on these materials are in the market including Decapeptyl® , Lupron Depot® and Sandostalin LAR®. For parenteral applications, these have been used simply as inert carrier vehicles. Preparation of various dosage forms were carried out by many methods, by simply incorporating the drug directly into the polymer matrix peptides, proteins and genetic and cell based drugs plays a greater role on the performance as the effective delivery of new drug therapies of the polymer platform
A wide variety of delivery systems have been developed for the purpose of prolonging the release and finally bioavailability of drugs to the body. Examples include the transdermal patch, oral dosage forms such as osmotic pumps and swellable hydrophilic polymer matrices and various types of polymer – based parenterals. There are also many limitations and associate with oral or transdermal administration and also poor drug stability in the GI tract, low drug to permiability etc.
One example of a nondegradable delivery system is Norplant® which is used successfully in prevention of pregnancy for up to five years. This system consists of 6 small, closed tubes made of a silicone rubber copolymer of dimethysiloxane and methylvinysiloxane and is implanted subcutaneously by scalpel incision. Each tube consists 36 mg of the progestin harmone levonorgestrel. The nondegradable delivery system like this at the terminal of the dose requires a secondary surgical procedure to retriene the implanted device from the body. Although Norplant is widely known to be safe and highly cost effective method of contraception, the demand for this product in united sates dropped since 1994. This is due to the difficulties associated with removal of the implanted rods along with the publicity created by these clinical problems.
II. HISTORY :
The use of biodegradable polymer in drug delivery grew from the search for polymers that could be employed as degradable sutures. Synthetic polymers such as poly (glycolic acid) were first developed in the 1950is and their poor hydrolytic stability made them unsuitable for permanent applications. But these materials are useful for applications as they could benefit from their ability to degrade in the presence of moisture. Examples include Dexon® and Vicryl® sutures prepared from poly (glycolic acid) and poly (lactide-co-glycolide). This led to the development of sustained release drug delivery formulations.
In 1970, Yolles et al reported the use of the poly (lactic acid) biodegradable system for delivery of the narcotic antagonist cyclazocine. At the same time a number of other drugs such as anticancer agents, steroids and other narcotic antagonists were reported.
More recently, the growth of biotechnology has led to the identification of many potent and powerful protein and powerful protein and gene based macromolecular drugs. Polymer drug interactions, processing conditions and the internal PH, temperature and moisture levels within the implanted device. Considerable achievements have been made in the development of biodegradable delivery systems of proteins and in particular bioactive peptides.
Over the past decade commercial success has been achieved using the polyester PLA and the various copolymers of PLG. Many biodegradable delivery systems have been developed for synthetic analogs of Luteinizing hormone releasing hormone (LHRH).
Sanders et al, demonstrated the release of nafarelin acetate for over 30 days from microsphere formulations of PLG with a 50:50 molar ratio of the lactide and glycolide monomers. The first such system came to market was Decapeptyl® (Ipsen Biotech), a microsphere depot formulation for treatment of prostate cancer. This product delivers 3.75 mg of an LHRH analog over a 30-day period.
Along with this microsphere formulations, Zoladex® (Astrazeneca) is a small implantable cylinder containing goserelin acetate in a polymer matrix of 50:50 PLG. This system delivers approximately 3.6 mg drug over a month period and is also for the treatment of prostate cancer. A second Zoladex system containing 01.6 mg goserelin has also been developed to release the drug for 3 months.
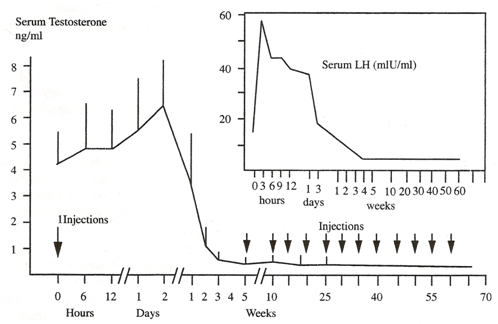
(Fig. 1) Serum LH and testosterone concentrations of 22 human subjects treated at 5 week intervals with the biodegradable depot formulation Decapeptyl (3 mg LHRH per dose).
The synthetic somatotropin analog octreotide acetate has been successfully formulated into a microsphere which composed of PLG, Sandostatin LAR® (Novartis) has been used for acromegaly treatment as well as the treatment of diarrhea and flushing episodes associated with metastatic earcinoid.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
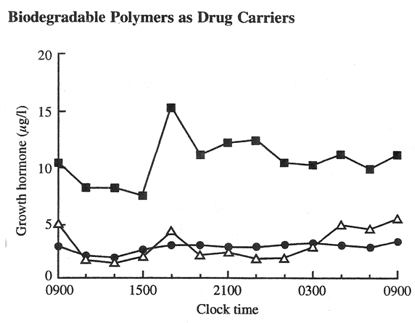
(Fig 2.) The influence of Sandostain LAR biodegradable depot formulation on the mean plasma growth hormone concentrations in humans. Plasma concentrations are shown over a 24 hours period 28 days after administration of a second monthly 20 m g dose of Sandostatin LAR, ·For comparison plasma concentrations are provided for untreated controls, and for patients receiving multiple daily subcutaneous octreotide injections, D
In December 1999, the first protein biodegradable depot formulation received regulatory approval. Nutropin Depot® (Genetech) contains somatotropin, a recombinant human growth hormone (rhGH) having a molecular weight of 22, 125 D within PLG microspheres. While many different polymer chemistries have been developed for drug delivery, only one class of polymer beside the polyesters has received regulatory approval.
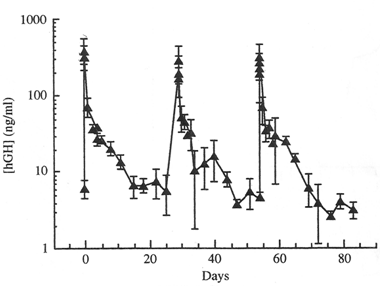
(Fig. 3) Serum human growth hormone (hGH) levels in immunosupressed rats receiving three monthly does of a biodegradable depot formulation containing 7.5 mg rhGH.
Gliadel® is a thin wafer containing the chemotherapeutic agent carmustine (BCNU) in a poly anhydride polymer matrix and was received approval conditionally in 1996. All these efforts are aimed at developing new polymeric materials designed specifically to overcome obstacles in drug delivery, bioavailability and stability. New engineering and processing techniques are developed to enhance drug delivery using the biodegradable polymers.
III) CLASSIFICATION OF BIODEGRADABLE POLYMERS :
a) Natural Polymers :
i) Proteins
Ex : albumin, collagen and gelatin
ii) Polysaccharides
Ex : cellulose, dextran, insulin and hyaluronic acid, starch.
b) Modified natural polymers
c) Synthetic polymers
a) Aliphatic poly (esters)
Poly (glycolic acid) and its co polymers
Poly (lactic acid) and its copolymers
Poly (E-caprolactone) and its copolymers
Poly (P-dioxanone)
Poly (hydroxybutyrate)
Poly (B-malic acid)
b) Poly (Phosphoester)
c) Polyanhydride
d) Polyphosphazene
e) Pseudo-amino acids
f) Poly (orthoesters)
II) B. Natural polymers and modified natural polymers :
Biopolymers or natural polymer are an attractive class of biodegradable polymers since they are :
· Derived from natural sources
· Easily available
· Relatively cheap
· Qualified for a number of chemical modification
The natural polymers can be proteins and polysaccharides in chemical origin. The modified natural polymers are natural polymers altered to improve their degradation profile. Generally labile polar functionalities are added to the polymer to enhance the degradability of the polymer. The extent and nature of polymer modification is vital as excess modification can hamper the biodegradation products. This modification of natural polymers is achieved by chemical modification and enzymatic alteration. The example of chemical modification is the cross lining of gelatin using formaldehyde. The chemical modification involves harsh condition to the enzymatic method.
The various potential natural and modified bio polymers for biomedical applications are discussed below :
Collagen :
Collagen, the primary structural protein occurs in the animal tissues, as aligned fibres in skin, connective tissue and the organic substance of bone. The prime function of collagen is to check tissue deformation and avoid mechanical failure. They offer the following advantages :
· Easy to isolate and purify in large quantities
· Biocompatible and non-toxic profile
· Well established physicochemical, structural and immunological properties.
· Amenable to easy processing to various forms.
Although it has been used as the basis of drug delivery systems, for example in the administration of medroxy progesterone and pilocarpine, it suffer from :
* Poor reproducibility
* Variability in drug release kinetics.
* Low mechanical strength and elasticity in vivo.
* In vivo swelling and resultant poor dimensional stability.
* Chances of triggering antigenic response.
Reconstituted collagen is perhaps the best known example of a natural polymer, which is subjected to degradation in vivo. It is utilized as a bioabsorbable suture, in prostheses, and as a wound dressing, and the rate of absorption can be varied from days to weeks by techniques such as succinylation or cross linking (with formaldehyde or glutaraldehyde). The mechanism of degradation is enzymatic and can be replicated in vitro with collagenase.
Albumin : Albumin is the major plasma protein component. In the human plasma it accounts for more than 55% of the total protein. They have been employed to design particulate drug delivery systems. The prime advantage includes their biodegradation into natural products, easy availability and absence of toxicity and antigenicity. Albumin microspheres have been employed to deliver many drugs including insulin, 1-norgestrel, sulphadiazine, triamcinolone, 5-fluorouracil etc. Basically, the albumin microspheres have been exploited for chemotherapy, as with them high local drug concentration can be achieved for a relatively longer period.
Various factors affecting the release of the drug from albumin microspheres are :
* Physicachemical properties and concentration of the drug.
* Interaction between albumin matrix and drug.
* Size and density of the microsphere.
* Nature and degree of cross-linking.
* Presence of enzymes and PH of the environment.
Typically the release pattern of drugs from albumin microsphers is biphasic. The initial release is followed by a comparatively slower first order release.
Fibrinogen :
Fibrin, the natural product resulting from the action of thrombin and fibrinogen, is available as commercially as a temporary be controlled by cross linking with formaldehyde. It is reported to be absorbed by polymorph nuclear digestive action.
Fibinogen microspheres can be prepared by an emulsification technique followed by thermal degradation. Doxorubicin, 5-Flurouracil and Adriamycin have been delivered with fibinogen microspheres.
Cellulose :
Cellulose is the most abundant polymer on the earth and these are large amount of cellulose product in our life. Cellulose triacetate one of the cellulose derivatives is a material for an artificial kidney machine. Cellulose is also used for food additives, clothes, paper, carrier and tablet media of medicines, materials of cosmetics and the filters of cigarettes.
Since cellulose is reproduced by nature with fixation of CO2 gas, recently it has been receiving keen interest as an environment friendly material. Now, a lot of research laboratories work on the research and development of cellulose. Cellulose is a linear homopolymer, consisting of B-1, 4-glycosidic linked D-glucopyranose units. Although, starch is also simple polysaccharide biosynthesised in nature, it has different 1, 4-glcosidic bonds structures (f2-1, 4-glycosidic bonds) from cellulose. The difference of only one structure causes a large difference between cellulose and starch. Cellulose forms robust inter and intrachain. H-bonds that do not exist in starch. The H-bonds are considered to be responsible for various properties of cellulose such as single-chain, conformation, stifness and solubility in solvent.
Chitin and Chitosan :
Chitin is a linear polycationic polymer of N acetyl – D-glucosamine (N-acetyl-2-amino-2-deoxy-D-glucopyranose) units linked by B-D(1-4) bonds. Chitin is highly insoluble in common solvents and has close resemblance to cellulose by having similar solubility profile and low chemical reactivity. An acetamide group may consider it as cellulose with hydroxyl at position C-2 replaced. The principal industrial sources of chitin are shell of shrimp, lobster and crab. Chitosan is a technologically important biomaterial. Chitin is the second most abundant natural polymer in the world after cellulose. Upon deacetylation, it yields the novel biomaterial chitosan, which upon further hydrolysis yields an extremely low molecular weight oligosacchalide. Chitosan possesses a wide range of useful properties Specifically, it is a biocompatible, antibacterial and environmentally friendly polyelectrolyte thus leading itself to a variety of applications including water treatment, chromatography, additives for cosmetics, textile treatment for antimecrobial activity, novel fibres for textiles, papers, biodegradable films, biomedical devices, and microcapsule implants for controlled release in drug delivery.
Chitin and chitosan are distinguished by their solubility profile in dilute aqueous acid solutions. The characteristic features of chitosan that render them suitable for pharmaceutical and biomedical applications are :
1. Pharmacological properties was antacid and antulcer activity, hypocholesterolemic action and wound healing properties.
2. Haemostatic and spermicidal properties owing to their ability to bind strongly mammalian cells by virtue of their polycationic character.
3. Presence of reactive functional group and cationic character opens up possibilities for their application in controlled drug delivery.
4. Favourable biological properties like biodegradability, biocompatibility and non-toxicity.
5. Has gel formation ability at low PH.
6. Chitosan matrix formation floats and swells in acidic medium.
For the past several years, chitosan has been primarily investigated as an excipient for oral drug formulations. Chitosan has been used as a direct tabletting agent, diluent, binder, lubricant and a potential disintegrant due to its water uptake properties. Addition of chitosan to conventional excipients leg. Mannitol, starch, lactose decreased their angle of repose and there by improved the fluidity of the powder mixtures.
Ulcerogenic drugs like aspirin can be effectively administered by chitosan as the later has gel-forming property at low PH and also has antiulcer and antacid properties. Gastric mucosal injury associated with diclofenac sodium can also be reduced with chitosan. Chitosan derivatives like chitosan glutamate facilitates paracellular drug delivery by opening epithelial tight junctions. Direct interaction of the cationic polymer with the negatively charged cell membrane permits this transport. Chitosan also holds immense promise for opthalmic delivery. The chitosan displays higher mucoadhesiveness in a natural or slightly alkaline PH. The film forming capacity of the chitosan can be employed for the development of contact lenses. It has been employed in ocular bandage lenses used as protective devices for acutely or chronically traumatised eyes.
Alginate :
Alginates are hydrophilic carbohydrates obtained from various species of brown seaweeds (pharphyceae) by the use of dilute alkali. Sodium alginate, the sodium salt of alginic acid is a polyuronic acid composed of B-D-mannuronic acid residues linked to that carboxylic group of each unit remains free while a glycosidic linkage shields the aldehyde group.
Alginates can be easily fabricated into particulate carriers. They are particularly beneficial as peptide carriers and other sensitive drug moieties. Since particulate carriers can be easily prepared in aqueous solution at room temperature. Alginate microspheres have been effectively used for oral delivery of vaccines due to benefits like :
1. They protect vaccines / antigens against degradation in gastro intestinal tract allowing stimulation of immune response with smaller amounts of vaccines / antigens.
2. They act as adjuvant
3. They bypass the stomach and thereby deliver the antigen to GALT.
II. B. ii) Synthetic polymer :
1.1 Aliphatic poly (ester)s : Among the degradable polymers identified the ester bond containing aliphatic polyesters are the most attractive and promising owing to their remarkable biocompatibiolity and versatility in terms of physical, chemical and biological properties. The prominent members of this class of polymers are :
Polymer Structure
Poly (glycolic acid) -(O-CO-CH2-)n
Poly (lactic acid) -(O-CO-CH(CH)3-)n-
Poly (E-laprolactone) -(O-CO-CH2)5-)n-
Poly (P-dioxanone) -(O-CO-CH2)2-O-CH2)n-
Poly (hydroxybutyrate) -(O-CO-CH(CH3)-CH2-)n-
Poly (B-malic acid) -(O-CO-CH(COOH)-CH2)n-
Out of these only a few polymer (eg : PLA, PGA, PCL and their copolymers) have reached the clinical stages as bioabsorbable drug delivery devices since in addition to biodegradable profile they have to meet other prerequisites for clinical use and commercialisation. The aliphatic polyesters can be synthesised by two methods, viz, polycodensation of bifunctional hydroxy acids and ring opening of polymerisation of cyclic ester monomers.
1.1 Polylactide and Copolymers (PLA) :
A delayed – release delivery system for water – soluble macromolecules was developed based on polylacetide. By coating a macromolecule, such as a protein, with PLA of different molecular weight and thickness, various delayed periods can be obtained. Such a delivery system is expected to be useful in delivering vaccines, which require occasional booster shots.
To retain the desirable feature of having the polymer break down into non-toxic residues in the body, cyclo (glycine-DL-lactic acid) can be copolymerised with DL-dilactide by ring opening techniques. The glycine renders PLA more hydrophilic and accelerates the degradation. The degradation mechanism of this glycine – PLA copolymer is bulk hydrolysis, and is autocatalyzed by the generated carboxylicesed groups.
1.2 Poly (lactide-glycolide) copolymers (PLGA) :
Among the biodegradable polymers, the most widely studied biodegradable synthetic polymers are polylactate (PLA) and polyglycolate (PGA) because they are eminently biodegradable and their degradation products are natural metabolites and therefore pose little toxic threat. They have been used usually as copolymers (PLGA), in every conceivable biomedical application starting with reabsorbable sutures, including burn dressings, asterial/vascular grafts, in bone repair, drug delivery systems, and other system of a temporary nature. The physical advantages of PLGA copolymers include strength and hydrophobicity. Their non toxic nature, biodegradability profile, biocompatible nature, was of fabrication attracted attention to its potential as excipients for drug delivery.
Polyglycolide (PGA) is the simplest linear aliphatic polyester. PGA was employed to develop first totally synthetic absorbable suture marketed. The copolymer composition and the mechanical and the degrdation properties do not have a linear relationship.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Properties of various Lactide / Glycolide Copolymers :
|
Polymer |
Melting Point |
Glass transition temperature |
Degradation time |
|
Poly (L-Lactide) |
172-174 |
60-67 |
18-24 |
|
Poly (DL-Lactide) |
None |
57-59 |
12-16 |
|
Poly (glycolide) |
220-225 |
35-40 |
6-12 |
|
DL-Lactide/ glycolide Copolymer (85:15) |
Amorphous |
45 |
5-6 |
|
DL-Lactide/ glycolide Copolymer (25:75) |
Amorphous |
60 |
4-5 |
|
DL-Lactide/ glycolide Copolymer (65:35) |
Amorphous |
45-50 |
3-4 |
|
DL-Lactide/ glycolide Copolymer (50:50) |
Amorphous |
45-50 |
1-2 |
The Lactide / glycolide polymer chains are cleaned by hydrolysis to the monomeric acid. They are eliminated in vivo through the kreb’s cycle, basically as CO2 and in the urine. The degradation rate at various body sites is more or less identical as the hydrolysis of these polymers is dependent only o the significant changes in temperature and PH and presence of catalysts.
Enzymatic is reported to play a vital role in the bioerosion of PLGA. In the early stages, when the polymer is in the glassy state little or no enzymatic involvement is evident but as the polymer acquires rubbery state and when soluble byproducts are released, the enzymes play significant role in biodegradation. The enzymes reported to effect degradation of PLGA include bacterial proteinase K, tissue esterases, pronase and bromelain.
1. The random hydrolytic clearage of the ester linkage leading to the reduction in molecular weight.
2. The onset of weight loss and changes in the rate of chain scission.
Lactide / Glycolide polymers branched with polyrinyl alcohol and dextran acetate show a monophasic and thereby uniform drug release profile in comparision to biphasic profile observed with linear molecules. The Lactide / glycolide polymers are useful excipients for targeting drug to the macrophages as they are biodegradable by monocytes macrophages within 78h. The drug release pattern from lactide / glycolide copolymer is a combination of initial leaching / diffusion followed by bioerossion of the matrix. The rate and duration of drug release is governed by polymer composition, drug / polymer ratio and size distribution pattern of microspheres.
1.3 Poly – E – Caprolactone (PCL) and its Copolymers :
Studies of PCL have provided a relative complete picture of the factors and mechanisms involved in the biodegradation of polyesters. PCL is a semi crytalline polymer (m.p. 630c) with low glass transition temperature (Tr-60 oc). It is soluble in benzene, chloroform, carbon tetra chloride, cyclohexanone and tolurence at room temperature. The earlier suggested use was as a component in biodegradation packaging to curtail environmental pollution.
The advantages of PCL as biodegradable controlled drug delivery systems include:
* Its slow degradation rate renders it suitable for use in long term (1 year) delivery systems.
* Biodegradability can be increased by copolymerisation.
* High permeability to large number of drug moieties
* Non-toxic profile.
* Its unique ability to form compatible blends with many other polymers.
The PCL has the unique ability to form compatible blends with other polymers. Thus the rate of drug release can be easily manipulated by formulating blends of PCL with cellulose propionate, cellulose acetate butyrate, poly lactic acid and poly lactic – co – glycolic acid. PCL and poly – 2 hydroxy ethyl methacrylate (PHEMA) have been used to prepare interpenetrating networks (1 PNs). The PCL and its copolymers permit high permeability and also exhibit a controllable induction period before the polymer is degraded, thereby offering way for designng diffusion controlled biodegradable systems. Rapid drug release rates from films of PCL and its copolymers with lactic acid are obtained. The release rates depend on the method of film preparation with compression – mailded films giving better release rates than the solution caste films.
Polyanhydrides :
This group was introduced specifically as a biodegradable polymer for controlled release systems. Based on its hydrophobic character and its labile hydropilic backbone, anhydride functional group, the polyanhydride was predicted to give a surface hydrolysable bioerodible drug delivery system. However, these polymers seem to gradually become less soluble on standing which is a clear disadvantage from a fabrication point of view. Examples of this class are poly (terephthalic anhydride), poly (bis(-paracarboxyphenoxy)propane anhydride), poly (sebacic anhydride).
Polyamides :
Among the synthetic polyamides like Nylon – 66(N66), Nylon 266, Nylon – 6 (N6), poly (E-laprolactum), Nylon 26 only those containing the naturally occuring alpha-L-amino acids, that is, poly-(alpha-L-amino acids), have been found to be biodegradable. Nylon 26 and Nylon 266 contain amino acid – glycine linkages and thus are biodegradable.
Polycarbonates :
Polycarbonates of interest in drug delivery systems are poly (ethylene carbonate) and poly (propylene carbonate). These are hydrophilic and degrade to physiologically tolerable products.
Hydrogels :
Hydrogels are polymeric materials that do not dissolve in water at physiological temperature and PH but swell considerably in aqueous medium. Hydrogels are of special interest in controlled release applications because of their safe tissue biocompatibility, the case with which the drugs are dispersed in the matrix and the high degree of control achieved by selecting the physical and chemical properties of the polymer network. Hydrogels consist of polymer chains cross – linked to each other to create a tangled mush structure, providing a matrix for the intrapment of drugs. These properties conducted a considerable research on hydrogels and their usage in controlled release technology.
In the studies, Risbud et al have reported, PH – sensitive chitosan – polyvinyl pyerolidinone hydrogels as a controlled release system that can be used in gastric environment.
In case of diseases involving peptic ulcers, it has been demonstrate that Helicobacter pylori is one of the major causative agents. This bacterium releases the enzyme urease, which converts urea into ammonia and bicarbonate, which aids in neutralising the acidic medium and allows the bacteria to colonise the gastric mucosa. Amoxicillin and metronidazole, which are effective in treating H pylori under in vitro conditions, score poorly when used to treat infections in an in vivo situation. The failure of these antibiotics has been proposed to be an outcome of sub-effective bactericidal concentrations available at the site and their instability following oral administration.
Cationic hydrogels with PH- sensitivity swelling properties have been proposed previously for stomach – targeted drug delivery systems. Such matrices can be used to provide adequate drug release in gastric (low PH) environments. Chitosan, a cation polysaccharide, is obtained by alkaline deacetylation of chitin, the principal exoskeletal component in crustaceans. Chitosan is reported to be non-toxic and bioabsorbable and has been explored for the release of many drugs. Risbud et al, have reported on a PH- sensitive chitosan – polyvinyl pycrolidinone, semi-interpenetrating polymer network based controlled release antibiotic delivery system that is well suited for use in a gastric environment.
This process has generated matrices with high porosity (pore diameter 39-2012.60 mm) that exhibited superior PH- dependent swelling properties which could be attributed to their porous nature. The increased swelling of hydrogels under acidic conditions was due to the protonation of the primary amino group on chitosan.
Polyurethanes (PURs) :
Polyurethanes are synthesised from polyols and polyisocyanates. They are classified roughly into two types, polyester PURs and polyether PURs, according to the type of polyols from which they were synthesised. Biodegradation has been reported to involve both bulk, hydrolysis and phayocytosis at the surface.
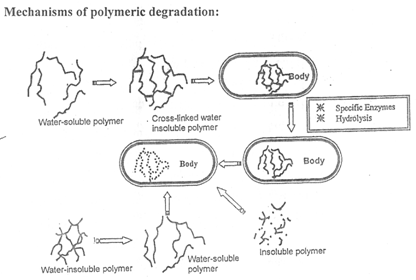
IV. POLYMER DEGRADATION MECHANISM :
The outstanding property of a biodegradable polymer is its degradation and erosion behaviour. The degradation is primarily the process of chain clearage leading to reduction in molecular weight. On the other hand, erosion is the sum of all the processes leading to the loss of mass from a polymer matrix. A polymer matrix can erode even without degradation. Conversely, it is possible that the polymer is degraded completely but is not eroded. The erosion of biodegradable polymer is crucial with respect in its behaviour as a carrier material for drug delivery.
Biodegradable controlled release systems offer the advantage of gradual biological elimination without a residual implant structure remaining. Biodegradable polymers, also called bioerodible or bioabsorbable polymers are synthetic or natural polymers, which hydrolyse in vivo. A large selection of biodegradable polymers are available as carriers for local drug delivery. Examples of these polymers are shown in table. These polymers degrade in the body at various time periods from a few days up to 2 – 3 years. These polymers degrade into non-toxic acids or alcohols that are readily excreted from the body. The in vivo elimination time is determined by the nature of the polymer chemical linkage, the solubility of the degradation products, the size, shape and density of the device, the drug and additive content, the molecular weight of the polymer, and the implantation site. Many of the site – specific applications of drugs are for periods of several weeks, requiring polymer carriers that degrade and are eliminated from the body soon after. It is believed that for many of the applications the polymer should be eliminated within 6 months after implantation. From the available polymers, Polyanhydrides, collagen and copolymers of lactide and glycolide are useful for short term drug release and device elimination in vivo. The degradation of polymer can be either bulk erosion (as in poly hydroxy esters) or surface erosion (as in poly anhydrides, poly (ortho esters)). Generally polymer degradation occurs in two phases.
1. In the first phase, water penetrates the bulk of the device and preferentially attacks the chemical bonds in the amophous phase leading to conversion of long chain polymers into shorter water – soluble fragments. As the water starts device fragmentation, the changes in the physical property become increasingly apparent.
2. In the second phase, there is a rapid loss of polymer mass due to enzymatic attack and fragment metabolization. In the bulk erosion the rate of water penetration into the device exceeds the rate at which polymer is converted into the water – soluble materials.
(Fig.4)
Common biodegradable polymer carriers :
|
Polymer |
Polymer Linkage |
Principal Degradation product |
Elimination time (months) |
|
Poly (lactic acid) |
-CO-O- |
Lactic acid |
12-24 |
|
Poly (lactic – co – glycolic acid) |
-CO-O- |
Lactic and Glycolic acid |
6-12 |
|
Poly (glycolic acid |
-CO-O- |
Glycolic acid |
2-4 |
|
Poly (caprolactone) |
-CO-O- |
Hydroxypentanoic acid |
18-24 |
|
Poly (hydroxybutylate) |
-CO-O- |
Hydroxybutyric acid |
18-24 |
|
Poly (Orthoester) |
-CO-O- |
Alcohols |
12-24 |
|
Poly(alkane anhydride) |
-CO-O-CO- |
Aliphatic diacids |
0-2-4 |
|
Gelatin Collagen |
-CO-NH- |
Amino acids |
0-2-1 |
|
Oxidized cellulose |
-CO-CH-O- |
Alcohols, CO2 |
0-2-1 |
|
Poly (phosphazene) |
-N=P- |
Phosphates, ammonia |
|
Elimination times vary depending on implant size and shape, density, implantation site and molecular weight.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
V. BIOEROSION MECHANISM :
The term polymer erosion is generally used to signify the conversion of an initially water – insoluble material to water – soluble one. This process can proceed with or without chemical degradation.
Bioerodible polymers can be reservoir or matrix devices. In a reservoir system, a polymer surrounds a core of a drug and the rate- limiting step in drug release is the diffusion of drug outward through the polymer. This rate can be controlled or adjusted by changing the nature of the bioerodible membrane. In a matrix system, drug is dispersed uniformly throughout a solid polymer. Drug release is a product of their diffusion through or erosion of the polymer. In order to the clinically useful, biodegradable polymers must also be biocompatible. The polymer and its degradation products must either be safely eliminated from the body or metabolised to non-toxic substances. These are three general mechanisms of polymer hydrolysis :
1. Erosion of cross-linked polymers with hydrolytically unstable crosslinks. Polymer chains are freed from the bulk matrix as the cross-links are hydrolysed. This mechanism is useful for drugs with low water – soubility or large macromolecules.
2. Solubilization of water-insoluble polymers by hydrolysis ionisation or protonation of a side group, without any significant change in polymer molecular weight. This mechanism is mainly used in topical or oral applications.
3. Erosion of water – insoluble polymers with labile back bone









