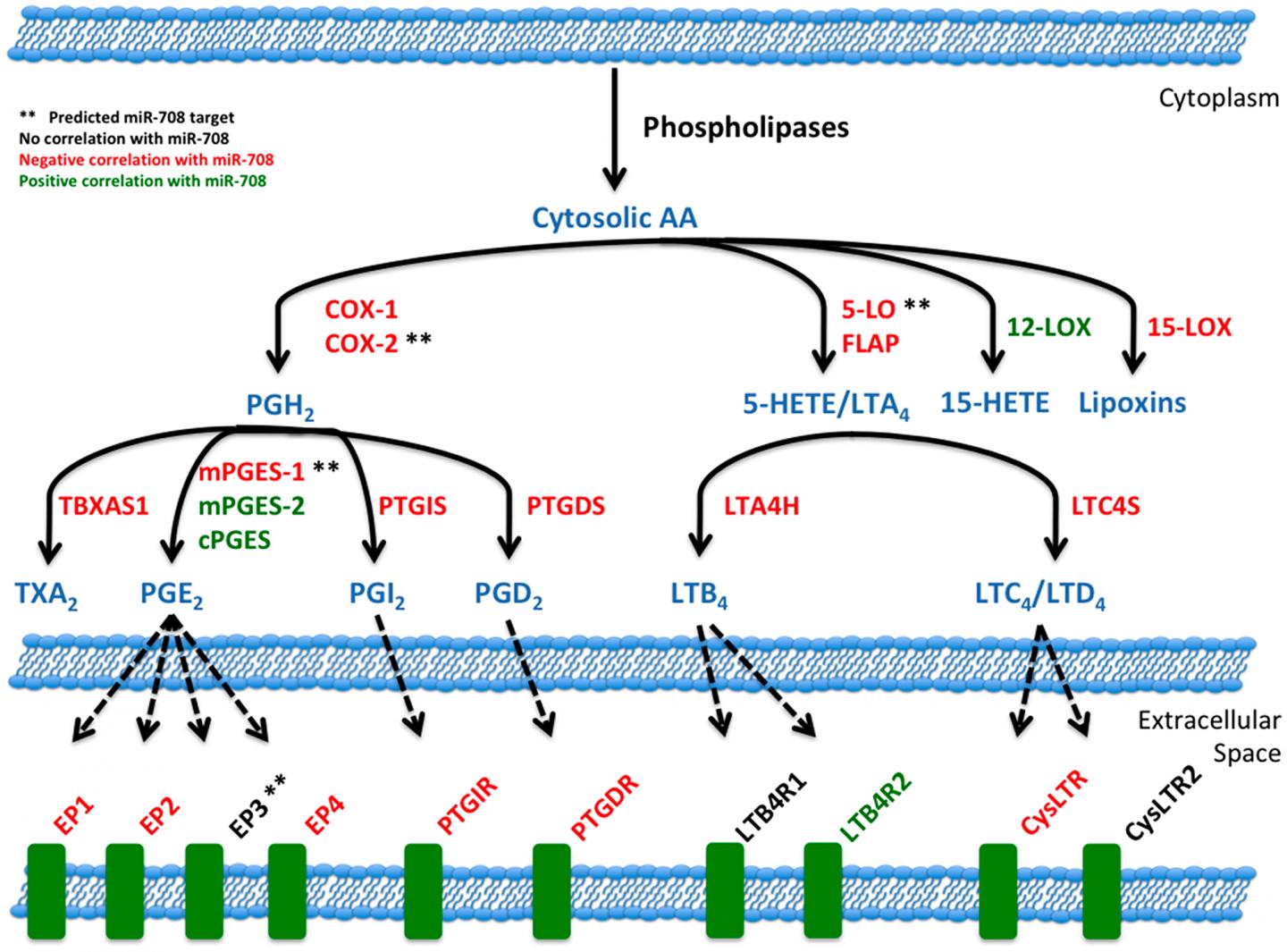
Caption : miR-708 and the arachidonic acid pathway. Illustration of miR-708's relationship to the AA signaling pathway. Blue lettering indicates short-lived intermediates and mature signaling molecules within the AA pathway. Red lettering illustrates a negative correlation between miR-708 and AA-related gene's mRNA expression in LUSC patients. Green lettering illustrates a positive correlation between miR-708 and AA-related gene's mRNA expression in LUSC patient. Black lettering illustrates no significant correlation. Solid black lines indicate metabolic steps, while dotted black lines indicate eicosanoid signaling to respective receptors. (**) Represents predicted miR-708 targets from microRNA. org and miRTarBase.
In this study, the authors show that high mi R-708 expression is associated with survival rates in lung squamous cell carcinoma patients. mi R-708 also represses PGE2 production by suppressing both COX-2 and mPGES-1 expression in lung cancer cells.
Moreover, mi R-708 decreases proliferation, survival, and migration of lung cancer cells, which can be partially attributed to mi R-708's inhibition of PGE2 signaling.
Lastly, they identify novel mi R-708 predicted targets and possible regulators of mi R-708 expression in lung cancer.
Collectively, these data demonstrate that dysregulated mi R-708 expression contributes to exacerbated PGE2 production, leading to an enhanced pro-tumorigenic phenotype in lung cancer cells.
Dr. Carol S. Lutz from The Department of Microbiology, Biochemistry, and Molecular Genetics at Rutgers Biomedical & Health Sciences, New Jersey Medical School, School of Graduate Studies in Newark New Jersey USA said, "Lung cancer is the most common cancer, with more than 2.09 million lung cancer cases worldwide in 2018."
More importantly, lung cancer is the deadliest cancer in the world, with more than 1.79 million lung cancer-related deaths in 2018.
Lung cancer is a collection of several distinct subtypes, with non-small cell lung cancer accounting for 85% of all lung tumors. mi R-708 also indirectly regulates the expression of genes involved in PI3K signaling, cell cycle progression, epithelial-mesenchymal transition, and cancer cell stemness.
In this study, the authors aim to decipher novel mi R-708 targets and suggest a solution to the controversy on whether mi R-708 is an oncogenic or tumor-suppressive mi RNA in lung cancer.
The Lutz Research Team concluded in their Oncotarget Research Paper that collectively, their findings suggest further study of mi R-708 in lung cancer.
The data paired with previous studies highlight a potential value for mi R-708 as a diagnostic in differentiating lung tumors, as well as a potential therapeutic intervention, particularly in lung squamous cell carcinomas.
Their work has identified novel tumor-suppressive mi R-708 functions by suppressing oncogenic PGE2 production through targeting of COX-2 and mPGES-1.
"Their work has identified novel tumor-suppressive mi R-708 functions by suppressing oncogenic PGE2 production through targeting of COX-2 and mPGES-1"
These findings could be the foundation for identifying novel mi R-708 targets, as well as regulators of mi R-708 expression in cancer.
Moreover, the study highlights the need to better understand lung cancer biology to improve the diagnosis and treatment of lung cancer, ultimately aiming to increase positive patient outcomes.













