
Scientists from France conducted a small clinical trials and reported that hydroxychloroquine, an antimalarial medicine, treatment is significantly associated with viral load reduction or disappearance in COVID-19 patients and they also reported that azithromycin along with hydroxychloroquine can faster the recovery.
In late December 2019, an outbreak of an emerging disease (COVID-19) due to a novel coronavirus started in Wuhan, China and rapidly spread in China and outside. The WHO officially declared it as pandemic in March 2020.
Philippe Gautret and Jean-Christophe Lagier along with their team reported in a clinical trial, "Hydroxychloroquine clinical safety profile is better than that of chloroquine (during long-term use) and allows higher daily dose and has fewer concerns about drug-drug interactions [14]. Our team has a very comprehensive experience in successfully treating patients with chronic diseases due to intracellular bacteria (Q fever due to Coxiella burnetii and Whipple’s disease due to Tropheryma whipplei) with long-term hydroxychloroquine treatment (600 mg/day for 12 to 18 months) since more than 20 years."
They had created three groups of patients which incuded as symptomatic, upper respiratory tract infection (URTI) when presenting with rhinitis, pharyngitis, or isolated low-grade fever and myalgia, and lower respiratory tract infections (LRTI) when presenting with symptoms of pneumonia or bronchitis.
Researchers observed in three to six days that hydroxychloroquine is efficient in clearing viral nasopharyngeal carriage of SARS-CoV-2 in COVID-19 patients, in most patients. A significant difference was observed between hydroxychloroquine-treated patients and controls starting even on day 3 post-inclusion.
They also suggest a synergistic effect of the combination of hydroxychloroquine and azithromycin. Azithromycin has been shown to be active in vitroagainst Zika and Ebola viruses and to prevent severe respiratory tract infections when administrated to patients suffering viral infection.
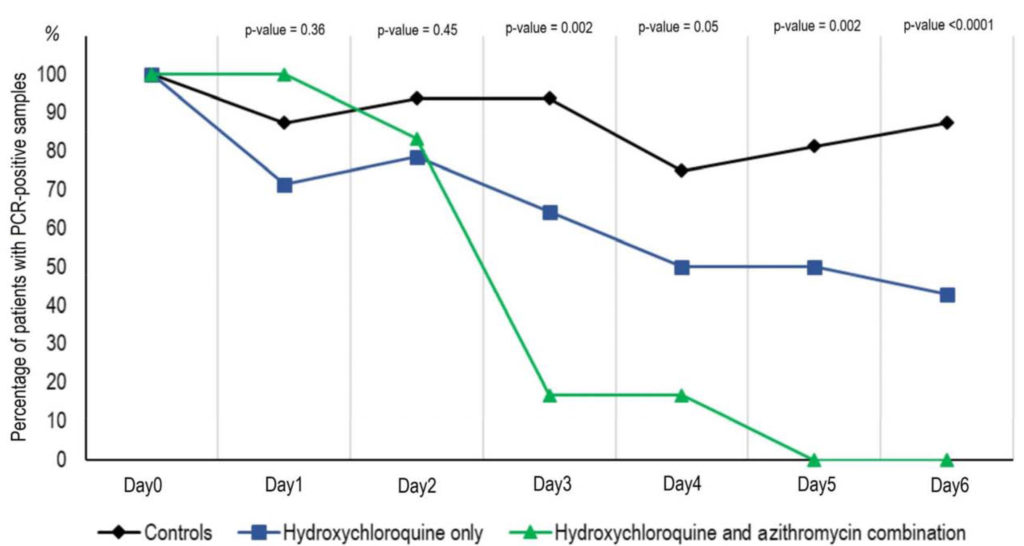
Percentage of patients with PCR-positive nasopharyngeal samples from inclusion to day6 post-inclusion in COVID-19 patients treated with hydroxychloroquine only, in COVID-19 patients treated with hydroxychloroquine and azithomycin combination, and in COVID-19 control patients.
This finding is published in International Journal of Antimicrobial Agents and author suggested that it should be further explored to know whether a combination is more effective especially in severe cases.
Reference: Gautret et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents – In Press 17 March 2020 – DOI : doi.org/10.1016/j.ijantimicag.2020.105949













