About Author:
Rajput Praveen Kumar*, Gahlot Kavita
College of Pharmacy,
Institute of Foreign Trade and Management (IFTM),
Moradabad (UP)
Abstract:
Rumex hastatus is the bushy shrub or undershrub 30-90 cm,high,occurring chiefly on dry rocks and hillsides on dry rocks and hillsides of western Himalayas from Kumaun to Kashmir,at altitudes between 300 and 2400 m . Rootstock woody; leaves 2.5-6.5 cm.long,hastate, thick and fleshy ; flowers pink or green tinged with pink, polygamous ,on terminal panicles;nut small,trigonous.
Leaves have a pleasant acid tase and can be eaten;they also serve as an ingredienr for chutney and pickles.Root and stem yield 21-23 percent tannin.
[adsense:336x280:8701650588]
Introduction:
BOTANICAL INFORMATION:
Rumex hastatus is a fairly common small shrub, growing on dry slopes, rocks and walls between 700-2500 m, typically in north-Indian hill stations. It is a bushy shrub with many ascending stems. Stems woody at base, leaves narrow and arrow shaped with a pair of narrow spreading basal lobe. Leaves vary a lot in length and breadth. The stems have numerous thin branches with terminal very slender clusters of distant whorls of tiny greenish pink or pinkish green flowers. Flowers very small, flower stalk lengthening in fruit. Leaves broadly triangular, stalked, fruit pinkish.
Flowering: June-October
Habitat of the herb: Wasteland, dry slopes and rocks to elevations of 2400 metres. Shady slopes or dry streambeds at elevations of 1000 - 2600 metres in Nepal.
Edible parts of Rumex hastatus:Tender young leaves and shoots - raw or cooked. A sour, acid flavour, it is eaten as a sorrel.
Propagation of Rumex hastatus: Seed - sow spring in a cold frame. When they are large enough to handle, prick the seedlings out into individual pots and plant them out in the summer. Division in spring.
Cultivation of the herb: Wasteland, dry slopes and rocks to elevations of 2400 metres. Shady slopes or dry streambeds at elevations of 1000 - 2600 metres in Nepal.
Root Anatomy: Epidermis in roots of R. hastatus, R. nepalensis, R. australe and P. maculosa was single layered. Length of the epidermal cells was maximum in R. hastatus and minimum in P. maculosa. . The many layered parenchyma compactly packed in all the 6 species. It was polygonal shape in R. nepalensis, R. australe and P. maculosa and spherical in R. dentatus, P. plebejum and R. hastatus. Length of the parenchyma was maximum in R. hastatus and minimum in P. maculosa. Width of the parenchyma was maximum in R. hastatus and minimum in P. maculosa. Sclerenchyma was present in R. hastatus and R. australe and was absent in other species. It was polygonal in R. hastatus and spherical in R. australe. . Endodermis was a single layered in R. hastatus, R. nepalensis, R. australe and P. plebejum. The cells were elongated in R. hastatus Length and widthof the cell was maximum in R. hastatus .Pericycle is absent in R. hastatus
Stem Anatomy: Epidermis in stem of R. hastatus, R. dentatus, R. nepalensis, R. australe and P. maculosa was single layered and two layered in P. plebejum. The cells were spherical in R. hastatus, oval in R. dentatus, R. nepalensis, R. australe and P. maculosa and elongated in P. plebejum The many layered parenchyma was compactly packed in al l species. It was polygonal in R. hastatus, R. australe, R. dentatus and P. maculosa and oval in R. nepalensis and P. plebejum. Length of the cell was maximum in R. hastatus and minimum in R. nepalensis. Width of the cell was maximum in R. hastatus and minimum in P. maculosa. Collenchyma was present only in R. hastatus and R. dentatus and R. nepalensis. It was spherical in shape . Single layered endosperm is present in R. hastatus, R. dentatus, R. nepalensis, P. maculosa and P. plebejum and was absent in R. australe. The cells were elongated in P. plebejum and oval in R. dentatus, R. nepalensis and R. hastatus. Pericycle was a single layered in all six specimens and was spherical in R. nepalensis, R. australe and P. maculosa and oval in R. hastatus, R. dentatus and P. plebejum. Length of the cell was maximum in R. hastatus and minimum in P. plebejum. Phloem was elongated in R. hastatus, . Pith was absent in R. hastatus
Petiole Anatomy: Petiole was absent in P. plebejum. Epidermis in stem of R. hastatus was two Layered Parenchyma in all species was compactly packed. It was polygonal in R. hastatus, R. dentatus, R. australe and P. maculosa and spherical in R. nepalensis. Endodermis was two layered in R. hastatus The cells were oval in R. dentatus, R. nepalensis and R. hastatus and spherical in P. maculosa. Pericycle was a single layered in all specimens and was spherical in R. nepalensis, R. hastatus and P. maculosa . Phloem was spherical in R. hastatus, R. dentatus and R. nepalensis and P. maculosa and oval in R. australe. Pith was absent in R. dentatus and R. hastatus.
Leaf Anatomy: In leaves epidermis was single layered in all six leaves. The cells were spherical in R. nepalensis and P. maculosa and elongated in R. hastatus, R. dentatus, R. australe and P. plebejum. Length of the cell was maximum in R. dentatus and minimum in R. hastatus. Width of the cell was maximum in R. dentatus and minimum in R. hastatus. Mesophyll was elongated. Collenchyma was present only in R. hastatus .Endodermis was single layered in R. hastatus. Pericycle was oval and single layered in R. hastatus. Xylem was oval in R. hastatus. Phloem was spherical in R. hastatus. Pith was absent from R. hastatus
Medicinal use of Rumex hastatus: The juice of the plant is astringent and is used in the treatment of bloody dysentery. The fresh tuber is chewed to relieve aches in the throat.The leaf extract of plant are applied on wounds and cuts to check bleeding. Plant is also believed to relieve from suffering of nettle sting. Root is laxative alternative, tonic, and anti rheumatic and can be used in skin disease
Known hazards of Rumex hastatus: Plants can contain quite high levels of oxalic acid, which is what gives the leaves of many members of this genus an acid-lemon flavour. Perfectly alright in small quantities, the leaves should not be eaten in large amounts since the oxalic acid can lock-up other nutrients in the food, especially calcium, thus causing mineral deficiencies. The oxalic acid content will be reduced if the plant is cooked. People with a tendency to rheumatism, arthritis, gout, kidney stones or hyperacidity should take especial caution if including this plant in their diet since it can aggravate their condition(7)
MATERIAL & METHODS:
Collection and Authentication of Plant Material: from Ranikhet region
Macroscopical charecters: as per methods described by Wallis.
Microscopical charecters: as per methods described by Wallis.
Ash values: as per Indian Pharmacopoeia (2007).
Extractive values: as per Indian Pharmacopoeia (2007).
Loss On Drying: (LOD)as per Quality control methods for medicinal plants material (WHO).
Preparation of extracts: as per methods ofHarbone
Phytochemical Screening: as per methods described by Khandelwal
PHARMACOGNOSTICAL STUDY:
Collection and Authentication of Plant Material
The plants of Rumex hastatus DC.were collected during the month of October from Chaubatia garden, Ranikhet and authenticated from National Botanical Reasearch Institute (NBRI). The photo documentation of this plant in natural habitat has been shown in below figures.

Fig.FLOWERING TWIGS of Rumex hastatus
Macroscopical Characters: As follows (Wallis T.E.)
1. Size Length 20-30 cm
Diameter 2-5 mm
2. Colour Greenish yellow
3. Surface character Smooth
4. Shape Regular branched
5. Fracture Short
6. Odour Characteristic
7. Taste Acrid

Fig: Stem of Rumex hastatus
Microscopical Characters: As follows. (Khandelwal K.R.)
(A) Transverse Section (T.S.): Shows the following structures.
Cork: Epidermis in stem was single layered. The were spherical in shape. Parenchymatous cell are in many layered and compactly packed. They are polygonal in shape.
Cortex: Collenchyma were present. It was spherical in shape.
Endodermis: Single layered
Starch: Simple and rounded in shape.
Xylem Vessels: They are oval in shape.
Pericycle: Pericycle was a single layered
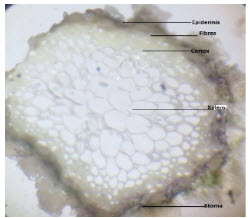
Fig. 1.1- T.S OF Rumex hastatus x 50
(B) Powder Characteristics:
Table: 1 Staining
|
S.No. |
REAGENTS |
OBSERVATION |
CHARACTERISTICS |
|
1 |
Phloroglucinol + conc. Hcl (1:1) |
Pink |
Xylem vessels, Xylem fibers, Trichomes |
|
2 |
Iodine solution |
Blue |
Starch |
|
3 |
Acetic acid |
Insoluble |
Calcium oxalate crystals |
|
4 |
Hydrochloric acid |
Soluble |
Calcium oxalate crystals |

Fig 1.2 –Starch grains X 100

Fig 1.3 –Fibres X 100
DETERMINATION OF ASH: As follows (WHO guidelines.)
Ash consists of inorganic radicals like phosphates, carbonates & silicates of sodium, potassium; calcium etc. is used to determine quality & purity of a crude drug.
The ash remaining following ignition of medicinal plant material is determined by different methods which measure total ash, acid insoluble ash, water soluble ash and sulphated ash.
The total ash method is designed to measure the total amount of material remaining after ignition. This includes both “physiological ash”, which is derived from the plant tissue itself. And “non-physiological ash”, which is the residue of the extraneous matter (e,g sand and soil) adhering to the plant surface.
Acid-insoluble ash is the residue obtained after boiling the total ash with dilute hydrochloric acid and igniting the remaining insoluble matter. This measures the amount of silica present especially as sand and siliceous earth.
PROCEDURES:
For determination of different ash values, the stem of R.hastatus was powdered. The powder was passed through sieve no. 40 and used as follows:
TOTAL ASH:
1 g air dried powder was placed in a previously ignited and tarred crucidly. The powder was spreaded in an even layer and ignited by gradually increasing the heat to 500 – 600 0C until it is white, indicating the absence of carbon. Then crucible was cooled in desiccators. The ash was weighed and percentage of total ash with reference to air dried powder was calculated. The total ash value is given in table.
ACID- INSOLUBLE ASH:
25 ml of hydrochloric acid (-70g/l) was added to the crucible containing the total ash & boiled gently for 5 minutes. The insoluble matter was collected on the ash less filter paper & washed with hot water until the filtrate is neutral. The filter paper was transferred to the original crucible and ignited to a constant weight. The residue was to cool in a suitable dessicator for 30 min. The ash was weighed without delay and percentage of acid-insoluble ash with reference to air dried powder was calculated. The acid-insoluble ash is given table no.3
WATER SOLUBLE ASH:
25ml of water was added to the crucible containing the total ash and boiled for 5 min. The insoluble matter was collected on the ash less filter paper and washed with hot water. The filter paper was transferred to the original crucible and ignited to a constant weight at a temperature not exceeding 450oC. The residue was allowed to cool in a suitable desiccator for 30 min. The weight of the residue in milligram was subtracted from the weight of total ash. The ash was weighed without delay and percentage of water soluble ash with reference to air dried powder was calculated. Water soluble ash value is given in table no.3
Table: 3 ASH VALUES:
|
S. No. |
ANALYTICAL PARAMETERS |
*ASH VALUE (% w/w) |
|
1. |
Total Ash |
12% |
|
2. |
Acid Insoluble ash |
1.5% |
|
3 |
Water Soluble ash |
3% |
*Average of three determinations
DETERMINATION OF EXTRACTABLE MATER:
This method determines the amount of active constituents extracted with solvents from a given amount of medicinal plant material. It is employed for material for which as yet no suitable chemical or biological assay exists.
METHOD:
Cold Maceration:
The stem of R.hastatuswas powdered. The powder was passed through sieve no. 20& used as follows:-
Ethanol-soluble extractive:
5 g of air dried coarse powder was macerated with 100 ml of ethanol of the specified strength in a closed flask for 24 hours, shaken frequently during the first 6 hours & allowed to stand for 18 hours. Therefore filtered rapidly taking precaution against loss of ethanol, evaporated 25 ml of the filtrate to dryness in a tarred fiat- bottomed shallow dish, dried at 105oc & weighed. The percentage of ethanol- soluble extractive was calculated with reference to the air dried drug. The ethanol-soluble extractive value is given in table.
Water-soluble extractive:
Proceed as directed for the determination of ethanol –soluble extractive, using chloroform water instead of ethanol. The water-soluble extractive value given in table.
Petroleum ether-soluble extractive:
Proceed as directed for the determination of ethanol –soluble extractive, using petroleum ether instead of ethanol. The petroleum ether- soluble extractive value is given in table.
Table: 4 Extractive Values:
|
S. No. |
ANALYTICAL PARAMETER |
*EXTRACTIVE VALUE(% w/w) |
|
1. |
Petroleum ether-soluble extractive |
2.4% |
|
2. |
Ethanol-soluble extractive |
3% |
|
3. |
Water- soluble extractive |
4 |
*Average of three determinations
LOSS ON DRYING: As follows(Khandelwal K.R.)
Loss on drying is the loss of weight expressed as percentage w/w resulting from water & volatile matter of any kind that can be driven off under specified conditions. He test is carried out on a well mixed sample of substance is in the form of crystals, reduce the size by rapid crushing to a powder.
An excess of water in medicinal plant materials will encourage microbial growth, the presence of fungi or insects & deterioration following hydrolysis. Limits for water content should therefore be set for every given plant material. This is especially important for materials that absorb moisture easily or deteriorate quickly in the presence of water.
Procedure:
1g of dried powder was accurately weighed &placed in a previously dried weighing bottle. The sample was dried in a oven at 100-1050c until two consecutive weighing do not differ by more than 5 mg. The loss of weight in mg per of air dried material was calculated.
Report:
The loss on drying was found to be 11% w/w.
Preparation of Extracts: As follows. (Harbone J.B.)
The dry stem were separated from the collected plant during the month of March 2011 and washed with tap water to remove the dust and adhering materials. Then they were dried under shade and powdered in a pulverizer. The powder was extracted with ethanol (90% v/v) using a Soxhlet apparatus. Then, extract was dried in distillation apparatus to remove the excess of solvent to get a semisolid mass and kept in desiccators for the studies. Then, the powder was dried to remove the alcohol.
Further, it was subjected to cold maceration process by using chloroform-water solvent mixture for two days. Then it was filtered and the filtrate was concentrated to get semisolid mass. Both the extracts were kept in desiccators for the further studies.
Characteristics of Leaf Extracts:
|
S. No. |
Extracts |
Colour |
%yield (w/w) |
|
1. |
Alcoholic extract |
Dark brown |
7% |
|
2. |
Aqueous extract |
Dark red |
5% |
Preliminary Phytochemical Screening:
Phytochemical screening tests were carried out for the aqueous and alcoholic extracts of R.hastaus stem for the presence or absence of various phytoconstituents (Khandelwal K.R.)
Test for Carbohydrates:
Molisch’s test: Take 2-3 ml extract; add few drops of alpha- naphtol solution in alcohol, shake and conc. H2SO4 from sides of the test tube. Violet ring is formed at the junction of two liquids.
Test for Gums
Hydrolyze test solution using dilute HCl. Perform Fehling’s or Benedict’s test. Red color is developed.
Test for Mucilage:
(a) Powdered drug material shows red color with ruthenium red.
(b) Powdered drug swells in water or aqueous KOH.
Tests for Proteins:
(a) Biuret test: Mix 3 ml extract with 4% NaOH and few drops of 1% CuSO4 solution. Violet or pink color appears.
(b) Millon’s tast: Mix 3 ml extract with 5 ml Millon’s reagent. White ppt. Warm ppt. turns brick red or the ppt. dissolves giving red colored solution.
(c) Xanthoprotein test: Mix 3 ml extract with 1 ml conc. H2SO4. White ppt. is formed. Boil. Precipitate turns yellow. Add NH4OH, ppt. turns orange.
Test for Amino acids:
Ninhydrin test: Heat 3 ml extract and 3 drops 5% Ninhydrin solution in boiling water bath 10 min. Purple or bluish color appears.
Tests for Steroid:
(a) Salkowski reaction: Take 2 ml of extract, add 2 ml chloroform and 2 ml conc. H2SO4. Shake well. Chloroform layer appears red and acid layer shows greenish yellow fluorescence.
(b) Liebermann-Burchard reaction: Mix 2 ml extract with chloroform. Add 1-2 ml acetic anhydride and 2 drops conc. H2SO4 from the side of test tube. First red, then blue and finally green color appears.
(c) Liebermann’s reaction: Mix 3 ml extract with 3 ml acetic anhydride. Heat and cool. Add few drops conc. H2SO4. Blue color appears.
Test for Fats and Oils:
(a) Solubility test: Oils are soluble in ether, benzene and chloroform, but insoluble in 90% ethanol and in water.
(b) Saponification test: Evaporate extract to get 10 ml oil. Add 25 ml 10% NaOH. Boil in boiling water bath for 30 min. Cool. Add excess Na2SO4 solution. Soap forms and rise to the top. Filter. To filtrate add H2SO4. Evaporate. Collect residue, it contains glycerol. Dissolve residue in ethanol. With ethanolic solution, perform following test:
(i) Take ethanolic solution, add few crystals of KHSO4. Heat vigorously. Pungent odour of acrylic aldehyde is produced.
(ii) Take ethanolic solution and add few drops of CuSO4 and NaOH solutions. Clear blue solution is observed.
Tests for Alkaloids:
Evaporate the aqueous and alcoholic extract separately. To residue, add dilute HCL. Shake well and filter. With filtrate, perform following tests:
(a) Dragendorff’s test: Take 2-3ml filtrates and add few drops Dragendorff’s reagent. Orange brown ppt. is formed.
(b) Mayer’s test: 2-3ml filtrates with few drops Mayer’s reagent gives ppt.
(c) Hager’s test: 2-3ml filtrates with few drops Hager’s reagent gives yellow ppt.
(d) Wagner’s test: 2-3ml filtrates with few drops Wagner’s reagent gives reddish-brown ppt.
Test for Tannins and Phenolic compounds:
2-3 ml of aqueous or alcoholic extract, add few drops of following reagent:
(a) 5% FeCl3 solution: deep blue-black color.
(b) Lead acetate solution: white ppt.
(c) Gelatin solution: white ppt.
(d) Bromine water: decoloration of bromine water.
(e) Acetic acid solution: red color solution.
(f) Potassium dichromate: red ppt.
(g) Dilute iodine solution: transient red color.
Tests for Glycosides:
Determine free sugar content of the extract. Hydrolyze the extract with mineral acid. Again determine the total sugar content of the hydrolyzed extract. Increase in sugar content indicates presence of glycoside in the extract.
Tests for Cardiac glycosides:
(a) Baljet’s test: A thick section shows yellow to orange color with sodium picrate.
(b) Legal’s test (for cardenolaids): Take aqueous or alcoholic extract, add 1 ml pyridine and 1 ml sodium nitroprusside. Pink to red color appears.
(c) Keller-Killiani test (for deoxysugers): Take 2 ml extract, add glacial acetic acid, one drop 5% FeCl3 and conc. H2SO4. Reddish brown color appears at junction of the two liquid layers and upper layer appears bluish green.
Test for Flavonoids:
(a) Shinoda test: Take dry powder or extract, add 5 ml 95% ethanol and few drops of conc. HCL and 0.5 g magnesium turnings. Pink color observed.
(b) Small quantity of residue, add lead acetate solution. Yellow colored ppt. is formed.
(c) Additionofincreasing amount of sodium hydroxide to the residue show yellow coloration, which decolorizes after addition of acid.
Test for Saponin glycosides:
(a) Foam test: Shake the drug extract or dry powder vigorously with water. Persistent foam observed.
(b) Hemolytic test: Add drug extract or dry powder to one drop of blood placed on glass slide. Hemolytic zone appears.
Test for Anthraquinone glycosides:
(a) Borntrager’s test: Take 3 ml extract, add dil. H2SO4. Boil and filter. Tocoldfiltrate, add equal volume benzene or chloroform. Shake well. Separate the organic solvent. Add ammonia. Ammonical layer turns pink or red.
(b) ModifiedBorntrager’s test: Take 5 ml extract, add 5 ml 5% FeCl3 and 5 ml dil. HCL and heated on water bath for 5 minute. Cool and add benzene or any organic solvent. Shake well. Separated organic layer, add equal volume dilute ammonia. Ammonical layer shows pinkish red color.
Tests for Volatile oils:
Take hydro distillate material. Separate volatile oil from distillate and perform the following tests:
(a) Volatile oils have characteristic odor.
(b) Filter paper is not permanently stained with volatile oil.
(c) Solubility test: Volatile oils are soluble in 90% alcohol.(5)
Table: 5:Preliminary Phytochemical Screening:
|
S. No. |
Chemical Category |
Alcoholic extract |
Aqueous extract |
|
1. |
Carbohydrates |
-ve |
-ve |
|
2. |
Proteins |
-ve |
+ve |
|
3. |
Steroid |
+ve |
-ve |
|
4. |
Fats and Oils |
+ve |
+ve |
|
5. |
Alkaloids |
+ve |
-ve |
|
6. |
Tannins and Phenolic compounds |
+ve |
+ve |
|
7. |
Glycosides (i) Cardiac (ii) Flavonoids (iii) Saponin (iv) Anthraquinone |
-ve -ve -ve +ve |
-ve -ve -ve +ve |
|
8. |
Volatile oils |
+ve |
+ve |
+ = Present - = Absent
Results & Discussion:
1. The plants of Rumex hastatus D Don. were collected from Ranikhet regions and authenticated for my project work. The present investigation deals with Pharmacognostical studies of stem of this plant.
2. Except few biological studies, not much work has been done on this plant. Therefore, It has been proposed to undertake the present work entitled ‘’Pharmacognostical Studies on Stem of Rumex hastatus D Don.’’
3. The macroscopic characters of stem including the length 4-8 cm & thickness 1-3 mm, rounded, flattened, curved, branched, green in color, characteristeric odour and bitter in taste.
4. In microscopic studies, the transverse sections of stem the epidermis as single layered..Parenchymatous cell are in many layered and compactly packed were found. Collenchyma were present
5. Simple and rounded starch grains are present.
6. In physicochemical studies, different parameters like fo; total ash, water-soluble, acid-insoluble 12%, 3%, 1.5%, w/w respectively; extractive values like petroleum ether, ethanol and water 2.4%,3%, 4% w/w respectively and loss on drying 11% w/w were observed.
7. Preliminary phytochemical analysis of aqueous and alcoholic extract was carried out for detection of different compounds. The aq extracts were found to be positive for the presence of protein, amino acid ,steroid, fats and oil, alkaloids, glycosides (Anthraquinone), tannin and phenolic compound and volatile oils. The alcoholic extracts were found to be positive for the presence of fats and oil, tannin and phenolic compound and volatile oils.
Conclusion:
The stem of Rumex hastatus D Don. belongs to the family Polygonaceae, were collected from Regional Research Institute (Ayurveda) , Ranikhet and authenticated &taken up for the present study.
The present work on “Pharmacognostical Evaluation on stem of Rumex hastatus D Don”, the following conclusion could be made. In Pharmacognostical Studies, macroscopic characters, microscopic characters and analytical parameters like Loss on Drying, Ash Values, and Extractive Values were determined. This will help for the correct identification of the plant for future investigation.
References:
1. Anonymous , “ The Wealth of India – Raw Material ”, Vol XV, 1st edition , CSIR , New Delhi,2003, pp 92
2. Anonymous (2007), “Indian Pharmacopoeia”, Vol – 1, Addendum, pp 78-79.
3. Anonymous (1998) , “ Quality Control Methods for Medicinal Plant Materials” , 1st edition , WHO Geneva , pp 3-4, 6-7.
4. Chopra R.N., Nayar S.L. and Chopra I.C. (2006) ‘Glossary of Indian Medicinal Plant’ Publication & Information Directorate, CSIR, New Delhi, Ist Edn., pp 252
5. Khandelwal K.R. (2003) “Practical Pharmacognosy”, 10th edition, Nirali Prakashan, Pune ,pp 149-157.
6. Wallis, T.E (1997) “Text book of Pharmacognosy”, 5th edition, CBS publishers, Delhi , pp 360- 363.
7. ISHFAQ HAMEED, FARRUKH HUSSAIN AND GHULAM DASTAGIR, Department of Botany, University of Peshawar, Pakistan.
Reference ID: PHARMATUTOR-ART-1039
FIND OUT MORE ARTICLES AT OUR DATABASE









