{ DOWNLOAD AS PDF }
ABOUT AUHTORS
M. Manasa Rekha*, T. Mubeena
Department of Pharmacy Practice,
Annamacharya college of Pharmacy,
Rajampet, Andhra Pradesh, India.
* manasarekharoyal@gmail.com
ABSTRACT:
Clinical pharmacy is defined as that area of pharmacy concerned with the science and practice of rational medication use. The pharmacist, along with the prescriber has a duty to ensure that patients are aware of the risk of side effects and a suitable course of action should they occur. With their detailed knowledge of medicine, pharmacists have the ability to relate unexpected symptoms experienced by patients to possible drug interactions of their drug therapy. The practice in clinical pharmacy also ensures that drug interactions are minimized by avoiding drugs with potential side effects in susceptible patients. Thus, pharmacist has a major role to play in relation to prevention, detection, and reporting drug interactions.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2492
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 5 Received On: 30/01/2017; Accepted On: 14/02/2017; Published On: 01/05/2017 How to cite this article: Rekha MM, Mubeena T;A study on role of Clinical Pharmacist in identification and reporting of drug interactions in Physciatric Ward in a Teritary Care Teaching Hospital; PharmaTutor; 2017; 5(5);70-75 |
INTRODUCTION:
Clinical pharmacy is defined as that area of pharmacy concerned with the science and practice of rational medication use. Clinical pharmacy is the branch of pharmacy in which pharmacists provide patient care that optimizes the use of medication and promotes health, wellness, and disease prevention. Clinical pharmacists care for patients in all health care settings but the clinical pharmacy movement initially began inside hospitals and clinics. Clinical pharmacists often work in collaboration with physicians, nurse practitioners and other healthcare professionals. The Clinical Pharmacist Stating explicitly that the clinical pharmacist cares for patients in all health care settings emphasizes two points: that clinical pharmacists provide care to their patients and that this practice can occur in any practice setting.
The Role of Pharmacist in Management of Drug Interaction
The pharmacist, along with the prescriber has a duty to ensure that patients are aware of the risk of side effects and a suitable course of action should they occur. With their detailed knowledge of medicine, pharmacists have the ability to relate unexpected symptoms experienced by patients to possible adverse effects of their drug therapy. The practice in clinical pharmacy also ensures that drug interactions are minimized by avoiding drugs with potential side effects in susceptible patients. Thus, pharmacist has a major role to play in relation to prevention, detection, and reporting drug interactions.
Management options of drug interaction include
Avoiding the combination entirely: For some drug interactions, the risk always outweighs the risk, and the combination should be avoided. Because drug classes are usually heterogeneous with regard to drug interactions (as described above) one can often select a no interacting alternative for either the object drug or the precipitant drug.
Adjusting the dose of the object drug: Sometimes, it is possible to give the two interacting drugs safely as long as the dose of the object drug is adjusted.
Spacing dosing times to avoid the interaction: For some drug interactions involving binding in the gastrointestinal tract, to avoid the interaction one can give the object drug at least 2 h before or 4 h after the precipitant drug. In this way, the object drug can be absorbed into the circulation before the precipitant drug appears.
Monitoring for early detection: In some cases, when it is necessary to administer interacting drug combinations, the interaction can be managed through close laboratory or clinical monitoring for the evidence of the interaction.
Provide information on patient risk factors that increases the chance of an adverse outcome: It is clear from the clinical experience of physicians and pharmacists as well as published studies that most patients who take interacting drug combinations do not manifest adverse consequences.
Improve computerized screening systems: It is clear that computerized drug interaction screening systems have not been as successful as one hoped
Excessive number of drug interactions on the systems: Many pharmacists find that computerized drug interaction screening systems detect a large number of DDIs of questionable clinical significance.
Drug class differences not handled correctly: Almost all drug classes interact heterogeneously, because individual members of a drug class are often not metabolized by the same cytochrome P450 isozymes or ABC (ATP-binding cassette) transporters as other members of the class. The statins are a good example, because simvastatin and lovastatin are extensively metabolized by CYP3A4, atorvastatin is moderately metabolized by CYP3A4, fluvastatin is metabolized by CYP2C9, and pravastatin and rosuvastatin are not metabolized by cytochrome P450 isozymes. Thus, combining all members of this drug class together is rarely justified when considering drug interactions.
Current Indian Scenario of Drug Interactions and its Management
The prescribing information for most drugs contains a list of potential drug interactions. Many of the listed interactions may be rare, minor, or only occur under specific conditions and may not be important. Drug interactions that cause important changes in the action of a drug are of the greatest concern. Drug interactions are complex and chiefly unpredictable. A known interaction may not occur in every individual. This can be explained because there are several factors that affect the likelihood that a known interaction will occur
These factors include differences among individuals in their:
• genes,
• physiology,
• age,
• lifestyle (diet, exercise),
• underlying diseases,
• drug doses,
• the duration of combined therapy, and
• the relative time of administration of the two substances (Sometimes, interactions can be avoided if two drugs are taken at different times)
Management
• Before starting any new prescription drug or over-the-counter drug, talk to your primary health care provider or pharmacist.
• Make sure to read the patient information handout given to you at the pharmacy.
• Check the labels of your medications for any warnings and look for the “Drug Interaction Precaution.” Read these warnings carefully.
• Make a list of all your prescription medications and over-the-counter products, including drugs, vitamins, and supplements. Review this list with all health care providers and your pharmacist.
• If possible, use one pharmacy for all your prescription medications and over-the-counter products.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Underlaying factors
The factors or conditions that predispose or favour the appearance of interactions include:
• Old age: factors relating to how human physiology changes with age may affect the interaction of drugs. For example, liver metabolism, kidney function, nerve transmission or the functioning of bone marrow all decrease with age.
• Polypharmacy: The more drugs a patient takes the more likely it will be that some of them will interact.
• Genetic factors: Genes synthesize enzymes that metabolize drugs. Some races have genotypic variations that could decrease or increase the activity of these enzymes.
• Hepatic or renal diseases: The blood concentrations of drugs that are metabolized in the liver and / or eliminated by the kidneys may be altered if either of these organs is not functioning correctly.
Drug dependent factors:
• Narrow therapeutic index: Where the difference between the effective dose and the toxic dose is small.
• Steep dose-response curve: Small changes in the dosage of a drug produce large changes in the drug's concentration in the patient's blood plasma.
• Saturable hepatic metabolism: In addition to dose effects the capacity to metabolize the drug is greatly decreased
Pharmacokinetic interactions
Modifications in the effect of a drug are caused by differences in the absorption, transport, distribution, metabolization or excretion of one or both of the drugs compared with the expected behaviour of each drug when taken individually.
Absorption interactions
Changes in motility
Some drugs, such as the prokinetic agents increase the speed with which a substance passes through the intestines.
PH: Drugs can be present in either ionised or non-ionised form, depending on their pKa (pH at which the drug reaches equilibrium between its ionised and non-ionised form).
Drug solubility: The absorption of some drugs can be drastically reduced if they are administered together with food with a high fat content.
Formation of non-absorbable complexes:
• Chelation: The presence of di- or trivalent cations can cause the chelation of certain drugs, making them harder to absorb.
• Binding with proteins: Some drugs such as sucralfate binds to proteins, especially if they have a high bioavailability.
Transport and distribution interactions
The main interaction mechanism is competition for plasma protein transport.In these cases the drug that arrives first binds with the plasma protein, leaving the other drug dissolved in the plasma, which modifies its concentration.
Metabolism interactions
Many drug interactions are due to alterations in drug metabolism
Enzymatic inhibition
If drug A is metabolized by a cytochrome P450 enzyme and drug B inhibits or decreases the enzyme's activity, then drug A will remain with high levels in the plasma for longer as its inactivation is slower. As a result, enzymatic inhibition will cause an increase in the drug's effect.
Enzymatic induction
If drug A is metabolized by a cytochrome P450 enzyme and drug B induces or increases the enzyme's activity, then blood plasma concentrations of drug A will quickly fall as its inactivation will take place more rapidly.
AIM:
The study aims to assess the role of clinical pharmacist in identification and reporting of drug interactions in psychiatric ward in a teritary care teaching hospital.
OBJECTIVES:
The Main Objective Of The Present Study Includes Preventing the medication related problems (Drug interactions.),Monitoring drug interaction, Identifying and minimizing medication related problems, Improving Patient safety initiatives, Providing better therapy to the large number of patients, Improving the Patient health related outcomes.
METHODOLOGY:
Study Design: It is a Prospective observational study.
Study Period: The Present study was conducted for a period of two months from November 2016 to Jan 2017.
Study site : The Present study was conducted in Psychiatric Department at Rajiv Gandhi Institute of Medical Sciences ( RIMS), Kadapa.
Sample size: The Patients admitted in hospital during the study period of two months it was 70 Patients.
Source of Data:
All the patients satisfying the inclusion criteria were selected from Psychiatric department in Rajiv Gandhi institute of medical sciences (RIMS) Government Hospital, Kadapa. All the required data was collected from patients through Patient representative interview and case sheets and treatment charts.
Inclusion criteria
• Patients with aging above 18 years.
• Patients having previous history of medical, medication problems.
• The Patients who are willing to participate in the study.
Exclusion criteria
• Patients who are not willing to Participate in the study.
• Pregnancy.
• Lactation.
• Cancer Patients.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS:
AGE WISE DISTRIBUTION OF MALE POPULATION: In this study total of 70 patients were enrolled in the study. The males population is 42.The age wise male Patients population ranges from the 0 Patients were in the age group of 10-20 years , 2 Patients were in the age group of 20-30 years( 4.76190%),15 patients were in the age group of 30-40 years ( 35.7142 %),18 patients were in the age group of 40-50 years ( 42.8571 %), 7 Patients were in the age group of 50-60 years( 16.66 %).
Table 1.1 Age wise distribution of male patients
|
Age in years |
Total number of Patients |
Percentage (%) |
|---|---|---|
|
10-20 |
0 |
0 |
|
20-30 |
2 |
4.76190 |
|
30-40 |
15 |
35.7142 |
|
40-50 |
18 |
42.8571 |
|
50-60 |
7 |
16.66 |
|
Total |
42 |
60 |
|
Mean ±sd |
|
P value <0.0001 |
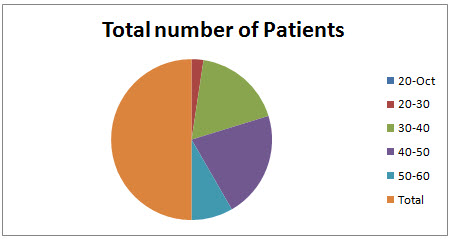
Fig 1.1 Age wise distribution of male patients
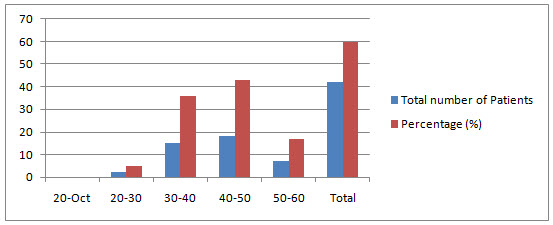
Fig 1.2 Age wise distribution of male patients showing percentage of distribution
AGE WISE DISTRIBUTION OF FEMALE POPULATION:
In this study total of 70 patients were enrolled in the study. The Females population is 28.The age wise Female patients population ranges from the 0 Patients were in the age group of 10-20 and 20-30 years, 7 Patients were in the age group of 30-40 years ( 25 %),13patients were in the age group of 40-50 years ( 46.42 %), 3patients were in the age group of 50-60 years( 10.71 %),4 patients were in the age group of 60-70 years ( 14.28 %),1 patients were in the age group of 70-80 years ( 3.57142 %).
Table 1.2 Age wise distribution of Female Patients
|
Age |
Total |
Percentage (%) |
|---|---|---|
|
10-20 |
0 |
0 |
|
20-30 |
0 |
0 |
|
30-40 |
7 |
25 |
|
40-50 |
13 |
46.42 |
|
50-60 |
3 |
10.71 |
|
60-70 |
4 |
14.28 |
|
70-80 |
1 |
3.57142 |
|
Total |
28 |
40 |
|
Mean ±sd |
|
P value <0.0001 |

Fig 1.3 Age wise distribution of Female patients.

Fig 1.4 Age wise distribution of Female patients showing percentage of distribution
Table 1.3 Showing The Various Medical Diagnosis Cases
|
VARIOUS MEDICAL DIAGNOSIS CASES |
TOTAL |
|
PARANOID SCHIZOPHRENIA |
8 |
|
SCHIZOPHRENIA |
13 |
|
DEMENTIA |
5 |
|
ALCOHOL DEPENDANCE SYNDROME CURRENTLY WITH DRAWL |
2 |
|
ANTI PHYSCOTIC DRUG INDUCED MOMENT |
4 |
|
MANIA WITH SCHIZOPHRENIA |
10 |
|
EPILEPSY WITH PHSYCHOSIS |
15 |
|
SCHIZOPHRENIA NOT RESPONDING TO MEDICATION |
3 |
|
MANIA |
5 |
|
EPILEPSY |
5 |
|
Total Cases |
70 |
SEVERITY OF DRUG INTERACTIONS
Total 64 drug interactions were screened during the study period.In which 17 (24.28571%) prescriptions having major drug interaction, 5 (7.1428571%) Prescriptions having minor drug interaction, 20 (28.57142851%) prescriptions having moderate drug interactions.
Table 1.4: Number and Severity of Drug interactions
|
Type of severity |
Number of drug interactions |
Percentage (%) |
|
Major |
17 |
24.28571 |
|
MODERATE |
20 |
28.57142851 |
|
MINOR |
5 |
7.1428571 |
|
Total |
42 |
60 |
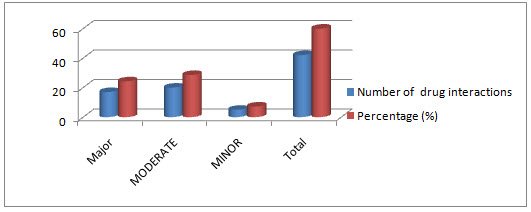
Figure 1.5 : severity of drug interactions
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
DISCUSSION:
Total 64 drug interactions were screened during the study period. In which 17 (24.28571%) prescriptions having major drug interaction, 5 (7.1428571%)Prescriptions having minor drug interaction, 20 (28.57142851%) prescriptions having moderate drug interactions.
CONCLUSION:
The present study concludes that clinical pharmacist play a key role in patient safety, Particlularly in Psychiatric department they are important for educating guiding the health care professionals related to safety use of medications. Even through drug related problems were founded but after reporting information to physicians we are minimizing harmful to Patient’s. In future clinical pharmacy services is one of the effective services in hospital to improve the quality of life of patient’s in the hospital. Implementing such a practices in the hospitals and country we can make the country and society becomes free of diseases.
REFERENCES:
1.*M. Manasa rekha ,A. Bharath kumar ---a prospective study on implementation of clinical pharmacy services to general medicine department in a teritary care teaching hospital published in journal of pharma research in ISSN: 2319-5622.
2.American College of Clinical Pharmacy. Pharmacotherapy 2008-28 (6), 816–817.
3. American College of Clinical Pharmacy.The Definition of Clinical Pharmacy. Pharmacotherapy 2008; 28(6):816-817.
4.Viktil KK, Blix HS. The Impact of Clinical Pharmacists on Drug-Related Problems and Clinical Outcomes. Basic Clin Pharmacol Toxicol 2008; 102(3):275-80.
5. Van den Bemt P M, Egberts T C , De Jong-van den Berg LT ,Brouwers J R Drug-Related Problems in Hospitalised Patients Drug Safety 2000 ; 22 (4): 321-333.
6. Blix HS, Viktil KK, Reikvam A, Moger TA, Hjemaas BJ, Pretsch P.The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals.Eur J Clin Pharmacol 2004; 60:651–658.
7. Roberts MS , Stokes JA , King MA, Lynne TA, Purdie DM.Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. Br J Clin Pharmacol 2001; 51:257-265.
8. Langebrake C, Hilgarth H. Clinical Pharmacist intervention inGerman University Hospital. Pharm World Sci 2010; 32:194-199.
9. Bruno Charpiat, Ornella Conort, Francoois-Xavier Rose Assessment of Clinical Pharmacists Interventions in FrenchHospitals: Result of Multicenter Study. Ann. Pharmacother 2008Jul; 42(7):1095-1103.
10. Alagiriswami B, Ramesh M, Parthasarathi G, BasavanagowdappaH. A Study of Clinical Pharmacist Initiated Changes in DrugTherapy in a Teaching Hospital. Indian J Pharm Pract 2009;1(2):36- 45.
11. Ganachari MS, Mahendra kumar BJ, Shashikala CW, fibrin M.Assessment of drug therapy intervention by Clinical Pharmacistin Tertiary care Hospital. Indian J Pharm Pract 2010; 3(3):22-28.
12. S Mangasuli, Padma, GM Rao. Clinical intervention: Apreliminary survey in a South Indian teaching hospital. Indian JPharmacol 2006 Oct;38(5):361-362.
13.Parthasarathi G, Ramesh M, Kumar JK, Madaki S. Assessment ofdrug related problems and clinical pharmacists ’ interventions inan Indian teaching hospital. J Pharm Prac Res. 2003; 33:272-74.
14. Anne Spinewine, Soraya Dhillon, Louise Mallet et al.Implementation of Ward-Based Clinical Pharmacy Services inBelgium-Description of the Impact on a Geriatric Unit. Ann.Pharmacother 2006 Apr; 40:720-728.
15. CJ Hawkey, S Hodgson, A Norman, TK Daneshmend, ST Garner.Effect of reactive pharmacy intervention on quality of hospitalprescribing. BMJ. 1990; 300:986-990.
16. ND Barber, R Batty, DA Ridout. Predicting the rate of physician-accepted interventions by hospital pharmacists in the UnitedKingdom.405.
17. G Parthasarathi, Karin nyfort-hansen, Milap C Nahata. A textbook of clinical pharmacy practice, second edition, Indcom Press, India, 2012, 2-18.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









