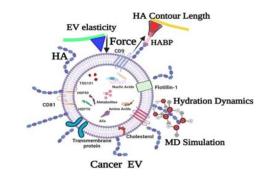
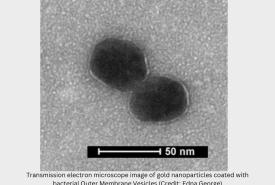
Pfizer and BioNTech seeks approval for Omicron Vaccine Booster in children
Pfizer Inc and BioNTech SE announced they have completed a submission to the U.S. Food and Drug Administration (FDA) requesting Emergency Use Authorization (EUA) of a 10-µg booster dose of the companies’ Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine for children ages 5 through 11 years of age.