A microencapsulation method, developed by OIST researchers, can help to overcome major challenges in pancreatic islet transplantation.
[adsense:336x280:8701650588]
Diabetes is one of the leading causes of death. Patients with type 1 diabetes have their insulin secreting cells destroyed by the immune system and require daily insulin injections. Pancreatic islet transplantation is an effective treatment that can dramatically reduce daily doses or even eliminate dependence on external insulin. Insulin producing cells are injected into a recipient liver. After an adaptation period they start to produce sufficient hormone needed by diabetic patients.
However, while the transplantation procedure itself has been greatly improved in recent years, collection, preservation, and transportation of these cells are still very challenging. Research published in Advanced Healthcare Materials by the scientists from the Okinawa Institute of Technology and Science Graduate University (OIST) in collaboration with the University of Washington and Wuhan University of Technology offers a solution for some of these problems.
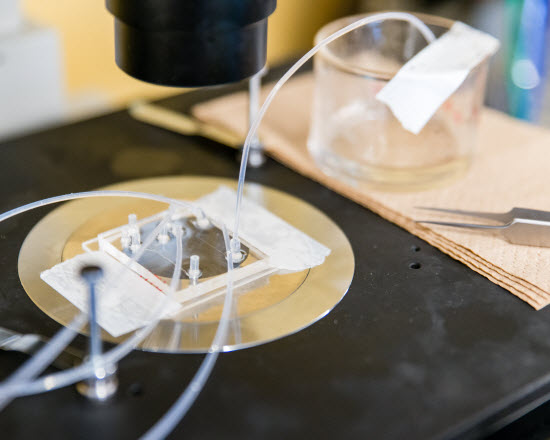
Image Credit: OIST | The Micro/Bio/Nanofluidics Unit at OIST has developed a novel microfluidic platform that allows to encapsulate small particles or cells with alginate capsules.
Production and secretion of insulin occur in the pancreas — an endocrine gland in the digestive system. Cells secreting insulin are clustered in pancreatic islets. Despite their crucial role in organismal wellbeing these islets comprise only a few percent of the pancreatic tissue. The islet transplantation does not require major surgical intervention and is often done under local anaesthesia. It is also cheaper and might be safer than transplantation of the entire pancreas. Unfortunately, so far, only human islets can be transplanted and their supply is but a trickle.
Cryopreservation, or deep freezing, is the method commonly used for the islet preservation and transportation. But it is not completely safe. One might think that storage at temperatures below -190°C is the most dangerous phase. However, the cells are very good at enduring it. It is the freezing process (-15 to -60°C) itself that poses the most challenges. As the cells are cooled, water in and around them freezes. Ice crystals have sharp edges that can pierce membranes and compromise cell viability. This also becomes problematic during thawing.
A multidisciplinary group of researchers led by Prof. Amy Shen, head of the Micro/Bio/Nanofluidics Unit at OIST, developed a novel cryopreservation method that not only helps to protect pancreatic islets from ice damage, but also facilitates real-time assessments of cell viability. Moreover, this method may reduce transplant rejection and, in turn, decrease use of immunosuppressant drugs, which can be harmful to patient health.
[adsense:468x15:2204050025]
The novel technique employs a droplet microfluidic device to encapsulate pancreatic islets in hydrogel made of alginate, a natural polymer extracted from seaweed. These capsules have a unique microstructure: a porous network and considerable amount of non-freezable water. There are three types of water in the hydrogel: free water, freezable bound water, and non-freezable bound water. Free water is regular water: it freezes at 0°C, producing ice crystals. Freezable bound water also crystallises, but the freezing point is lower. Non-freezable bound water does not form ice due to the strong association between water molecules and the hydrogel networks. Hydrogel capsules with large amounts of non-freezable bound water protect the cells from the ice damage and reduce the need for cryoprotectants — special substances that minimise or prevent freezing damage and can be toxic in high concentrations.
Another innovation, proposed by the group, is the use of a fluorescent oxygen-sensitive dye in hydrogel capsules. The porous structure of the capsules does not impede oxygen flow to the cells. And this dye functions as a real-time single-islet oxygen sensor. Fluorescence indicates whether cells are consuming oxygen and, therefore, are alive and healthy. It is a simple, time-efficient, and cheap method of assessing viability, both of individual islets or populations thereof.
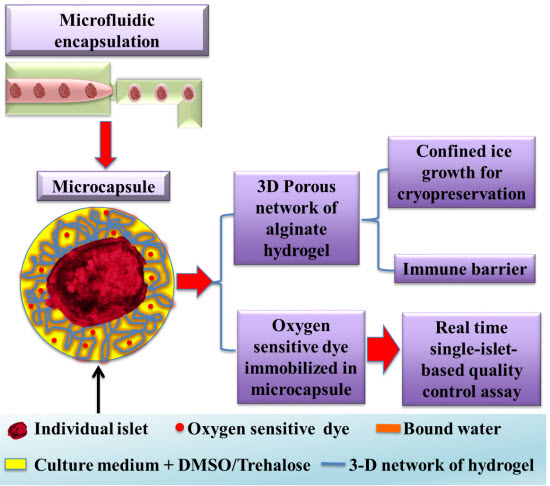
Image Credit: OIST | Schematic representation of the pancreatic islet cryopreservation method developed by a multidisciplinary group of researchers led by Prof. Shen.
Islet encapsulation reduces the risk of rejection of transplanted cells by the recipient. The hydrogel capsule allows small molecules, e.g. nutrients and islet secretions, to pass through the membrane easily, but prevents direct contact between implanted islets and host cells. Encapsulation also may prevent an attack on transplants by the autoimmune response that destroyed the patient’s own islets in the first place.
The microencapsulation method can help to overcome some major challenges in pancreatic islet transplantation, including the scarcity of available islets and the lack of simple and reliable control methods, especially for individual islet assessment. It offers hope to patients suffering from type 1 diabetes to return to a “normal” life, free of insulin injections.
Source: Okinawa Institute of Technology and Science Graduate University (OIST)













