Multiple Vacancies in Kusum Healthcare Pvt. Ltd | M.Pharm, M.Sc, B.Pharm

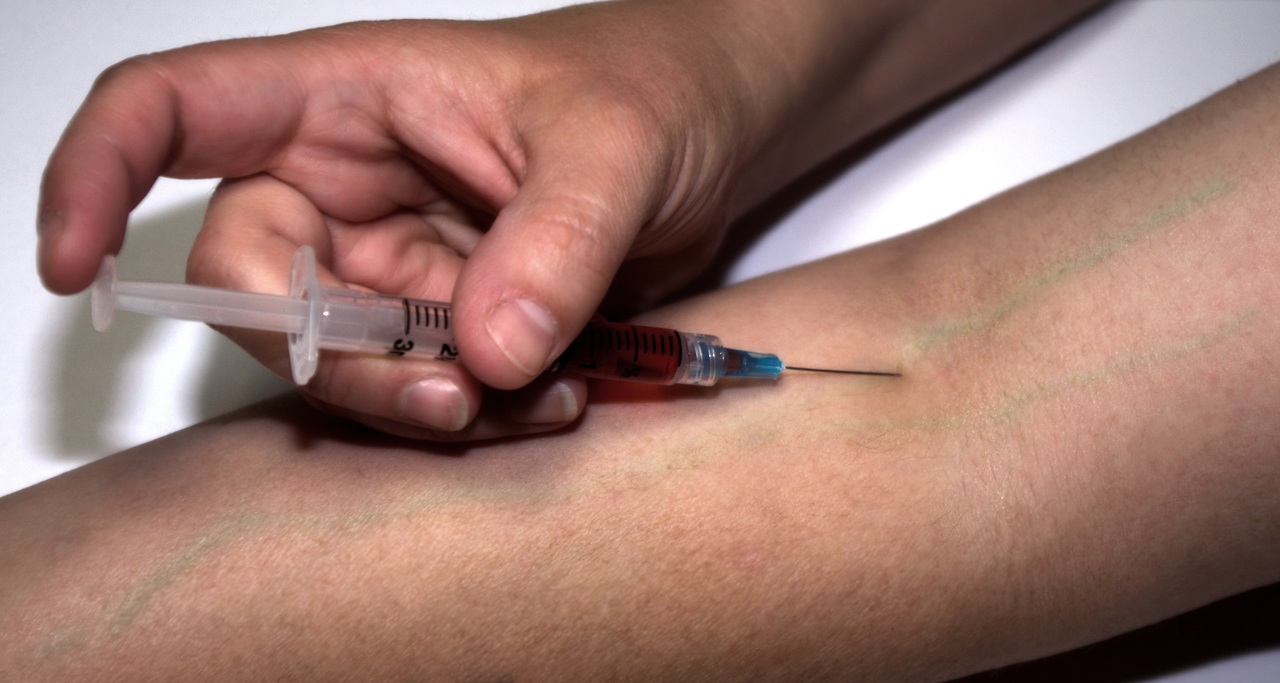
Cipla Limited announced the launch of remdesivir under its brand name CIPREMI. The U.S. FDA issued an Emergency Use Authorization (EUA) to Gilead Sciences Inc. for emergency use of remdesivir for the treatment of hospitalized 2019 coronavirus disease (COVID-19) patients. It is the only U.S. FDA approved Emergency Use Authorisation (EUA) treatment for adult and paediatric patients hospitalized with suspected or laboratory confirmed COVID-19 infection. In May, Gilead Sciences Inc. extended a voluntary non-exclusive license to Cipla to manufacture and market Cipla’s generic version of remedisvir called CIPREMI.
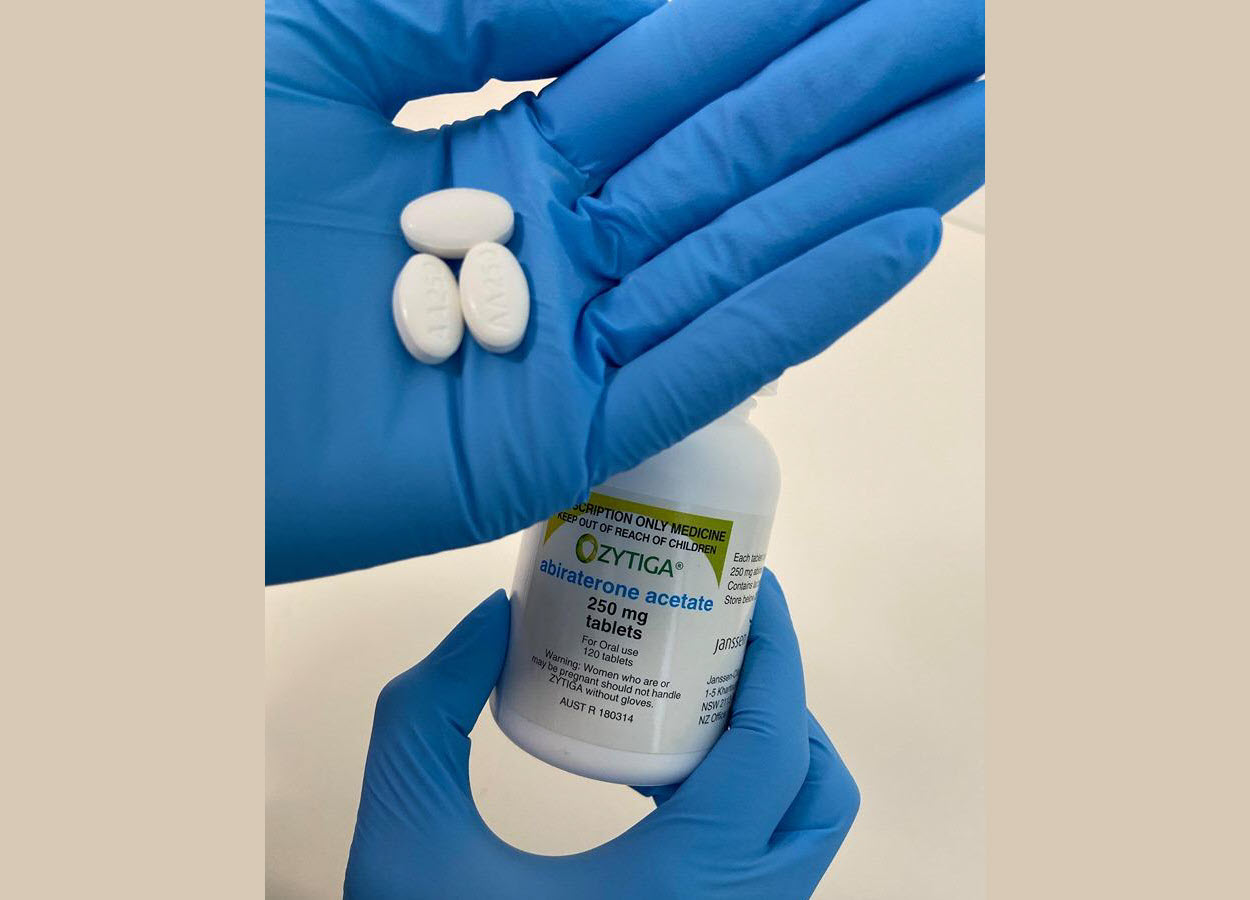
A novel formulation of the prostate cancer drug abiraterone acetate - currently marketed as Zytiga - will dramatically improve the quality of life for people suffering from prostate cancer, as pre-clinical trials by the University of South Australia show the new formulation improves the drug's effectiveness by 40 per cent.
Caption : A novel formulation of the prostate cancer drug abiraterone acetate - currently marketed as Zytiga - will dramatically improve the quality of life for people suffering from prostate cancer. Credit : Hayley Schultz/UniSA