 About Authors: - Tiwari1 Bhrigu Narayan*, Mr. Khatri1 Pankaj, Dr. Ali2 Julfikar.
About Authors: - Tiwari1 Bhrigu Narayan*, Mr. Khatri1 Pankaj, Dr. Ali2 Julfikar.
1 . Department of Pharmacognosy Gyan Vihar School of Pharmacy S.G.V. University ,Jaipur (Rajasthan)
2. Department of Biotechnology, School of Science S.G.V .University, Jaipur (Rajasthan)
Abstract
Tissue culture(often called micropropagation) is a special type of asexual propagation where a very small piece of tissue (shoot apex, leaf section, or even an individual cell) is excised (cut-out) and placed in sterile (aseptic) culture in a test tube, petri-dish or tissue culture container containing a special culture medium.Several industries and nurseries have standardized protocols for the multiplication of ornamentals like orchids genera, carnation, roses, chrysanthemum and plantation crop like banana and cardamom..The compounds responsible for the memory enhancing effects of Bacopa monniera are triterpenoid saponins called bacosides .In the Ayurvedic Materia Medica, Bacopa has been recognized for its brain enhancement characteristics. Bacopa monnieri, a member of the Scrophulariaceae family, is a small, creeping herb with numerous branches, small oblong leaves and light purple flowers. In India and the tropics it grows naturally in wet soil, shallow water and marches. Brahmi (Bacopa monnieri) (Linn) familyScrophulariaceaeis also known as Madhyarasayana in Ayurveda as it increase mental-clarityand brain stimulating action It also possesses anti-inflammatory, analgesic, antipyretic, epilepsy, insanity, anticancer and antioxidant activities, saponins such as bacosides A, B, C and D which are the active triterpenoid principles and known as "memory chemicals".Only a very limited research has been carried out on the plant, under the present study assumes singular significance and it is supposed to contribute a great deal to the existing literature. The present paper highlights the morphogenic response of various auxins and cytokinins on Bacopa monniera.
[adsense:336x280:8701650588]
INTRODUCTION
Tissue culture(often called micropropagation) is a special type of asexual propagation where a very small piece of tissue (shoot apex, leaf section, or even an individual cell) is excised (cut-out) and placed in sterile (aseptic) culture in a test tube, petri-dish or tissue culture container containing a special culture medium1.Tissue culture industry in the world has potential to grab a sizable chunk of the growing international market for production of secondary metabolite of medicinal important, flowers and other plants and earn valuable foreign exchange .The most viable technology in plant tissue culture today is the clonal / micropropagation of elites plants2. Several industries and nurseries have standardized protocols for the multiplication of ornamentals like orchids genera, carnation, roses, chrysanthemum and plantation crop like banana and cardamom.
Bacopa monnieri is a vegetatively propagated medicinal plant, which is endangered3. It is legendary for its diversity of usage. In the Ayurvedic Materia Medica, Bacopa has been recognized for its brain enhancement characteristics. Bacopa monnieri, a member of the Scrophulariaceae family, is a small, creeping herb with numerous branches, small oblong leaves and light purple flowers. In India and the tropics it grows naturally in wet soil, shallow water and marches. The herb can be found at elevations from sea level to altitudes of 4,400 feet and is easily cultivated if adequate water available. Flowers and fruits appear in summer and the entire plant is used medicinally. According to scientists at the Central Drug Research Institute in Lucknow, India, certain memory chemicals in Bacopa, called bacosides A and B, help repair damaged neurons by enhancing proteins involved in the regeneration of neural-cell synapses . The compounds responsible for the memory enhancing effects of Bacopa monniera are triterpenoid saponins called bacosides7 In a report to the Scientific Advisory Committee to the cabinet (SAC-C), Govt. of India, Technology Information, Forecasting and Assessment Council (TIFAC) 8has mentioned 45 medicinal plant species and specifically recommended 7 plants for immediate attention9
They are as follows- 1. Aloe vera (Ghrita Kumari) 2. Bacopa monnieri (Brahmi) 3. Centella asiatica (Mandukparni, Gotukola), 4. Rawolfia serpentine (Sarpagandha), 5. Catharanthus roseus (Periwinkle) 6. Taxus bacata 7. Artemisia annua
CHEMICAL CONSTITUENTS
Various chemical constituents were found in brahmi like alkaloids, saponins,sterol etc.
Alkaloids10,11: Brahmine, herpestatine, and a mixture of three bases.
Saponins: Saponin such as bacosides A, B, C, and D which are the active triterpinoid principles and known as “memory chemicals”12,13,14.
Table No. 131-34
|
S.No. |
Name of chemical |
Mol.Formulla |
Melting point |
|
1. |
Monnierin |
C5H82021-3H20 |
116-170 |
|
2. |
Bacoside –A |
C4H6.013.4H20 |
2500 |
|
3. |
Bacoside –B; |
C4H680,3.5H20 |
2030 |
|
4. |
Herpestatine |
C 34H46N206 |
116-170 |
|
5. |
Aglycone |
C30H4804 |
235-370 |
Fig:1- Bacoside - A
Fig:2 - Bacoside A3(P)
MATERIALS AND METHODS
COLLECTION OF PLANT MATERIALS:
(a) AREA OF COLLECTION:
The plant of Bacopa monnieriwere collected from the garden of National Instituteof Ayurveda, Jaipur(Rajasthan) India. The collected plant parts were kept under green house condition for further study.
(b) TIME OF COLLECTION:
The plants of Bacopa monnieri were collected in the month of July 2009 in morning time.
AUTHENTICATION:
The plant was authenticated by a botanist in Department of Botany, University of Rajasthan, Jaipur. A voucher specimen (RUBL20881) has been kept in herbarium in Department of Botany, University of Rajasthan, Jaipur.
CHEMICALS AND INSTRUMENTS
Instruments: Laminor air flow, Digital electronic weighing balance, pH meter, Magnetic stirrer, Autoclave, Hot air oven, Distilled water assembley, Heating burner, Culture room etc.
Glass-wares:Conical flask of various size, Glass rod, Spatula, Beaher of various size and other common glassware were the basic apparatus and instruments used for the study.
Chemicals: M.S. Media chemicals (CDH & Hi Media). Laboline(Thermo electron L.L.S. Mumbi,India), Agar type-1(CDH Laboratory Reagent ,New Delhi) andHCl, chloral sodium-hydroxide, Myoinositol, Sugar were procured from CDH Laboratory Reagent (P)Ltd,New Delhi., India.
METHODS
CULTURE MEDIA AND CONDITION
Murashige and Skoog’s medium was used for the cultivation of Bacopa monnieri L. at in -vitro condition. The MS medium was prepared by adding required amounts of stock solutions and final volume was made up withdistilled water, the composition of the MS medium was given in (Table2). The pH of the medium was adjusted to 5.7- 5.8 using 1 N NaOH/KCl. About 50 ml of the medium was poured into culture bottles. Bottles with MSmedium autoclaved at 121°C for 20 min. at 15 lbs pressure and transferred to the media storage room where they were kept under aseptic condition for further experiment.
Table 2: Composition ofMurashige and Skoog (MS Medium)a
|
S.No. |
Essential element |
Concentration in stock solution (mg/l) |
Concentration in medium (mg/l) |
|
|
Macroelementsb |
|
|
|
|
NH4NO3 |
3300 |
1650 |
|
|
K NO3 |
3800 |
1900 |
|
|
CaCl2.2H2O |
8 800 |
440 |
|
|
MgSO4.7H2O |
7 400 |
370 |
|
|
KH2PO4 |
3 400 |
170 |
|
2. |
Microelementsc |
|
|
|
|
KI |
166 |
0.83 |
|
|
H3BO3 |
1240 |
6.2 |
|
|
MnSO4.4H2O |
4460 |
22.3 |
|
|
ZnSO4.7H2O |
1720 |
8.6 |
|
|
Na2MoO4.2H2O |
50 |
0.25 |
|
|
CuSO4.5H2O |
5 |
0.025 |
|
|
CoCl2.6H2O |
5 |
0.025 |
|
3. |
Iron sourcec |
|
|
|
|
FeSO4.7H2O |
5560 |
27.8 |
|
|
Na2EDTA.2H2O |
7460 |
37.3 |
|
4. |
Organic supplementc |
|
|
|
|
Myoinositol |
20 000 |
100 |
|
|
Nicotinic acid |
100 |
0.5 |
|
|
Pyridoxine-HCl |
100 |
0.5 |
|
|
Thiamine-HCl |
100 |
0.5 |
|
|
Glycine |
400 |
2 |
|
5. |
Carbon sourced |
|
|
|
|
Sucrose |
Added as solid |
30 000 |
EXPLANT PREPARATION AND STERILIZATION:
Excised single segments of wereBacopa monnieri used as explants materials from disease free shoots of in-vitro plants bearing similar phenotypic expression. Excision was carried out when the stem was green. Single nodal cutting from stem were standardized to about 1.0-1.5 cm length and 0.3-0.5 cm of stem circumference. All leaves were detached. Following excision, the nodal segments were placed under running tap water for about an hour, after which the container was changed and the explants washed with a few drops of teeepol and tween 20 for about three minute with continuously agitation. The explants were the rinsed thoroughly with distilled water. In laminar flow, a diluted solution of 0.01% Hgcl2 w/v was prepared in a sterile container and explants was rinsed with HgCl2.The explants were then rinsed of three times with sterile distilled water for about 5 minutes each time and subsequently placed in sterile Petri dishes.
INITIATION OF CULTURE/CALLUS INDUCTION
Surface sterilized explants were transferred aseptically to sterile glass plate. Then undesirable and dead portions of both basal and the top portion of the explants were removed. The nodal explants were placed in an erect position in the culture bottle containing MS medium with the help of sterile forceps. Then lid was closed carefully and sealed with Klin film. The same procedure was used for all the explants. The culture bottles were kept in the growth room at 25±2°C, with a photoperiod of 16 h daylight and8 h night breaks under the cool white fluorescent light
ESTABLISHMENT OF CULTURES
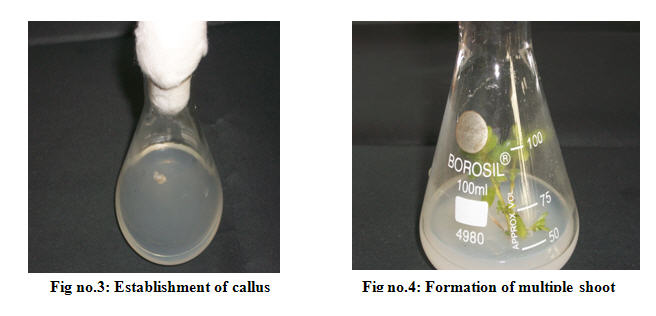
The explants with bud proliferation cultures were transferred to culture tubes containing fresh MS medium After 21-25 d of incubation the initiated plants were taken out from the culture bottle and transferred into fresh semi-solid MS media. Then the bottles were keptin culture room at 25±2°C for 8-16 h of day and night under the low temperature with white fluorescent light. After experimental days, the full matured culture was obtained and they were further subcultured in MS medium supplemented with different plant growth hormones at different concentrations for regeneration of shoots (Fig. No.3)
AUXILLARY SHOOT PROLIFERATION
The stem explants were inoculated on MS medium supplemented with different plantgrowth regulators at different concentrations and multiplication of shoots were carried out by repeated sub-culturing in MS medium. Multiple shoots and cluster were transferred from the culture bottle to a sterile glass plate and the debris parts were plate and the debris parts were transferred to the multiplication media with 0.2 -1mg/l BAP, 0.2 - 1mg/l 2,4-D, 0.2 - 1mg/l IAA, 0.2 -1mg/l BAP+2,4-D, 0.2 -1mg/l BAP+NAA as growth regulators. These culture bottles were incubated at 25±2°C. These steps were repeated at every 25-30 d intervals and shoot induction rate was observed.(Fig. No.4)
ROOTING OF THE SHOOTS
The surface sterilized explants were cut into small pieces. For root induction, MS basal medium was supplemented with IAA 0.2 -1 mg/l and IBA 0.2 -1 mg/l and the initial pH of the medium was adjusted to 5.6-5.8. The explants were implanted aseptically on the culture media. All the cultures were maintained in culture room at 25±2°C for 8-16 h of day and night break under the white fluorescent light. After incubation the root induction rate was observed. Triplicates were maintained in each treatment
EFFECT OF GROWTH REGULATORS ON SHOOT INUCTION
The MS medium was supplemented with the plant growth regulators like auxin (IAA, 2,4-D & NAA) and cytokinin(BAP) at 40 different combinations. The combinations and their concentrations were mentioned in Table 3. The explants were inoculated at appropriate condition in culture bottles, and the shoot induction rate was observed, triplicates were used for each treatment.
Table 3:Different growth regulators for shooting response of B. monnieri L
|
Growth regulators
|
Conc.(mg/l)
|
Shoot explants |
Leaves explants |
|||||
|
|
|
Shoot formation (%)
|
Shoot length (cm) |
Shoot formation (%)
|
Shoot length (cm) |
|||
|
BAP |
0.2 |
20 |
0.58±1.09 |
8 |
0.34±0.17 |
|||
|
0.4 |
20 |
0.65±00 |
10 |
0.43±0.18 |
||||
|
0.6 |
25 |
0.83±0.5 |
10 |
0.54±0.01 |
||||
|
0.8 |
50 |
1.63±00 |
40 |
1.25±0.89 |
||||
|
1.0 |
80 |
3.17±0.12 |
70 |
2.25±0.11 |
||||
|
2,4-D |
0.2 |
25 |
0.89±0.12 |
20 |
0.85±0.08 |
|||
|
0.4 |
30 |
1.65±0.11 |
25 |
1.56±0.11 |
||||
|
0.6 |
50 |
2.68±0.11 |
50 |
2.35±0.18 |
||||
|
0.8 |
80 |
2.92±0.22 |
60 |
2.50±0.22 |
||||
|
1.0 |
85 |
4.71±1.12 |
75 |
4.60±0.27 |
||||
|
IAA |
0.2 |
30 |
0.87±0.3 |
30 |
0.72±0.33 |
|||
|
0.4 |
50 |
1.56±0.13 |
50 |
1.25±0.61 |
||||
|
0.6 |
70 |
2.65±0.61 |
65 |
2.03±0.62 |
||||
|
0.8 |
85 |
3.47±0.89 |
80 |
3.01±0.99 |
||||
|
1.0 |
90 |
4.95±0.9 |
90 |
0.85±0.65 |
||||
|
BAP/2,4-D |
1.0±0.2 |
50 |
0.95±0.89 |
50 |
1.01±0.98 |
|||
|
1.0±0.4 |
60 |
1.72±0.89 |
55 |
1.85±0.98 |
||||
|
1.0±0.6 |
70 |
2.15±0.11 |
70 |
2.05±0.89 |
||||
|
1.0±0.8 |
90 |
2.95±0.99 |
80 |
3.25±0.33 |
||||
|
1.0±1.0 |
95 |
4.90±00 |
95 |
4.25±0.33 |
||||
|
0.2±1.0 |
40 |
0.89±0.19 |
40 |
0.43±0.4 |
||||
|
0.4±1.0 |
50 |
1.65±0.41 |
40 |
0.83±0.69 |
||||
|
0.6±1.0 |
70 |
2.85±0.41 |
50 |
1.98±0.99 |
||||
|
0.8±1.0 |
90 |
3.89±0.99 |
70 |
2.98±0.11 |
||||
|
1.0±1..0 |
95 |
5.38±0.89 |
80 |
4.81±0.66 |
||||
|
BAP/NAA |
1.0±0.2 |
40 |
2.38±0.11 |
40 |
1.33±0.33 |
|||
|
1.0±0.4 |
40 |
2.84±0.99 |
50 |
2.65±0.62 |
||||
|
1.0±0.6 |
70 |
3.35±0.99 |
65 |
2.76±0.28 |
||||
|
1.0±0.8 |
75 |
4.35±0.18 |
75 |
3.50±0.11 |
||||
|
1.0±1.0 |
90 |
5.83±0.66 |
90 |
3.95±0.65 |
||||
|
0.2±1.0 |
50 |
2.25±0.66 |
40 |
2.33±0.62 |
||||
|
0.4±1.0 |
60 |
2.38±0.25 |
50 |
2.75±.062 |
||||
|
0.6±1.0 |
65 |
4.25±0.99 |
60 |
3.50±0.89 |
||||
|
0.6±1.0 |
75 |
3.75±0.99 |
70 |
4.03±0.33 |
||||
|
0.8±1.0 |
75 |
4.90±0.89 |
70 |
4.50±0.99 |
||||
|
1.0±1.0 |
80 |
5.38±0.99 |
85 |
5.25±0.99 |
||||
NAA=1-naphthylacetic acid,
2, 4 -D=2,4-dichlorophenoxyacetic acid,
BAP=6-benzylaminopurine,
IAA= Indole-3-acetic acid.
EFFECT OF GROWTH REGULATORS ON ROOT INDUCTION
MS basal medium was supplemented with different concentrations of auxin (IAA) 0.2 -1mg/l and IBA 0.2 -1mg/l and pH of the medium was adjusted to 5.6-5.8. The explants were implanted on the culture media. All the cultures were maintained at culture room at 25±2°C for 8-16 h of day and night break under the cool white fluorescent light and the root induction rate was observed.Table 4
Table 4: Effect of growth regulators on in vitro rooting of Bacopa monnieri L
|
Growthregulators |
Conc.(mg/l) |
Average no. of roots |
Root length (cm) |
|
IAA |
0.2 |
2.80±0.69 |
1.50±0.51 |
|
0.4 |
3.40±0.98 |
2.10±0.96 |
|
|
0.6 |
4.40±0.98 |
3.00±1.21 |
|
|
0.8 |
6.00±0.98 |
4.01±0.51 |
|
|
1.0 |
7.00±0.98 |
5.21±0.58 |
|
|
IBA |
0.2 |
1.81±0.62 |
2.11±0.85 |
|
0.4 |
2.63±0.62 |
3.00±1.21 |
|
|
0.6 |
4.12±0.62 |
4.50±0.96 |
|
|
0.8 |
5.01±0.62 |
5.23±0.89 |
|
|
1.0 |
5.81±0.62 |
6.99±0.89 |
IAA=Indole-3-acetic acid, IBA= Indole-3-butyric acid
EFFECT OF GROWTH REGULATORS ON CALLUS INDUCTION
The plant growth regulators such as auxins and cytokinins were supplemented into MS medium at 25 different combinations and callus induction rate was observed inTable 5.
Table 5:Effect of growth regulators on callus formation of Bacopa monnieri L
|
Growthregulators |
Conc. (mg/l) |
Callus formation (%) |
|
NAA |
0.2 |
20 |
|
0.4 |
50 |
|
|
0.6 |
75 |
|
|
0.8 |
80 |
|
|
1.0 |
90 |
|
|
2,4-D |
0.2 |
40 |
|
0.4 |
50 |
|
|
0.6 |
50 |
|
|
0.8 |
90 |
|
|
1.0 |
95 |
|
|
BAP |
0.2 |
25 |
|
0.4 |
40 |
|
|
0.6 |
60 |
|
|
0.8 |
70 |
|
|
1.0 |
80 |
|
|
BAP+2,4-D
2,4-D+BAP |
1.0+0.2 |
50 |
|
1.0+0.4 |
70 |
|
|
1.0+0.6 |
70 |
|
|
1.0+0.8 |
75 |
|
|
1.0+1.0 |
90 |
|
|
0.2+1.0 |
40 |
|
|
0.4+1.0 |
40 |
|
|
0.6+1.0 |
50 |
|
|
0.8+1.0 |
70 |
|
|
1.0+1.0 |
80 |
NAA=1-naphthylacetic acid, 2, 4 -D=2,4-dichlorophenoxyacetic acid,
BAP=6-benzylaminopurine
Transplantation with soil mixture
The rooted plantlets were taken out from the culture bottles and washed with water to remove the excess medium. The plantlets were kept under mist chamber for10-15 d. Then, it was transferred to 10 different soil mixtures with various ratio/combinations such as humus rich soil alone as a control, soil with vermi-compost (4:1 &4:2), farmyard manure (4:1 & 4:2) in sterile polythene bags. The small holes were made in the polythene bag for proper air circulation and removal of excess water. After 3 weeks.(Fig. No.5)
Transplantation to cultivation field
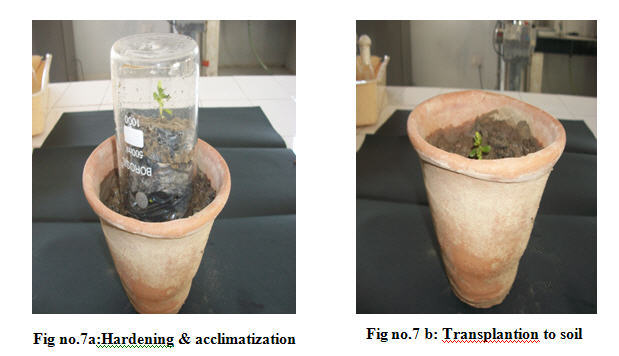
The rooted plantlets from the polythene bags were transferred to various cultivation field soil types including red soil, clay soil and sandy soil and water was poured at regular intervals for the growth of the plants. While transfer, the polythene bags were cut using sterile blade for better rooting into the soil. After 45 d, the plants from various soil types were uprooted, washed and the shoot and root length were measured using meter scale.(Fig. No.7)
EFFECT OF GROWTH REGULATORS ON INDUCTION OF SHOOT BUDS AND MULTIPLICATION
The present study was investigated the effect of various plant growth regulators (PGR) on shoot induction in 42 different combinations of which better results was observed in MS medium containing the following combinations of PGRs such as 0.2 -1mg/l BAP, 0.2 - 1mg/l 2,4-D, 0.2 - 1mg/l IAA, 0.2 -1mg/l BAP+2,4-D, 0.2 -1mg/l BAP+NAA. Remarkably, the MS medium containing 1 mg/I BAP, 1 mg/I BAP+2,4-D was showed excellent shoot formation followed by1mg/l BAP+NAA and1mg/l NAA . In this combination, the shooting response was observed as100% and maximum shoot length was recorded as 4.90±00cm after 3 weeks. Similarly from leaf explants, excellent shoot formation was observed in 1 mg/l BAP+2,4-D followed by 1 mg/l BAP+NAA containing MS medium and maximum shoot length was recorded as 5.25±0.99 cm followed by 4.25±0.33 when compared with other growth regulators combinations (Table 3). The moderate level of shoot formation was observed with the other growth regulators. Similar type of work has been reported by. MS media containing different concentrations and combinations of growth regulators were found to promote multiple shoots from both nodal and shoot tip explants. Multiple shoots start arising from nodes after 18-22 d of inoculation. Growth regulator combinations of BAP (1.0 mg/l) +2,4-D (1.0 mg/l) was producing maximum number of shoots and longer shoot length (4.90±00 cm) from nodal segments. Thus, it has been reported that the plant growth regulators greatly influence the shoot induction but it is varied depends upon the selected plant species used. (Fig no.6)

EFFECT OF GROWTH REGULATORS ON ROOT INDUCTION
The plant growth regulators not only control the shoot bud formation but also influence the root and callus induction. In accordance with this, the effect of IAA and IBA on root induction was carried out. Among them maximum number of roots (7.00±0.98) and root length(5.21± 0.58 cm) of the plants were noted in the MS medium containing 1.0 mg/l of IAA and 1.0 mg/l of IBA with number of roots (5.81±0.62) and root length(6.99± 0.89 cm) respectively. The maximum number roots (7.00±0.98) were produced when the medium supplemented with IAA similarly maximum root length (6.99± 0.89 cm) contributed by IBA at 1.0 mg/l on MS medium. The other concentrations of IBA and IAA could be response moderate to minimum level (Table 4).The root induction of plant gradually decreased with increasing concentration of auxin except 0.2, 0.4, 0.6, 0.8, 1.0 mg/l IAA and IBA respectively. The rooting was not obtained on auxin omitted medium.
EFFECT OF PLANT GROWTH REGULATORS ON CALLUS INDUCTION
Production of callus and its subsequent regeneration are the primary steps in crop plant to be manipulated by biotechnological means and to exploit somaclonal variation. In the present study remarkable callus induction rate was observed with nodes followed by leaves were used as explants and the appearance of B.monnieri L. callus was globular and pale yellow in colour. The explants were enlarged within 12-14 d of inoculation however callus formation was started after 15-20 d. Rapid callus growth (95%) response was observed in the MS medium with 1.0mg/l, 2,4-D and 1.0 mg/l NAA individually and BAP 1.0 mg/l with 2,4-D 1.0 mg/l , 2,4-D 1.0 mg/l + BAP 1.0 mg/l and in combinations of growth regulators. Minimum callus formation rate (10%) was noted in the MS medium containing 0.02 mg/l of NAA. The effect of growth regulators on callus formation was presented in Table 4. It has also been reported greatest callus formation observed from nodal explants ofBacopa cultured on MS medium containing 1.0 mg/l 2,4-DHence, the present and previous findings revealed that the callus induction was greatly influenced by growth hormones namely auxin or cytokinin in the nutrient media depending on the source of explants. Among the different factors influencing callus induction and regeneration genotype of the plants used, incubation condition and composition of nutrient media are the major factors that decide thein vitro raised cultures. (Fig no.3)
TRANSPLANTATION OF BACOPA MONNIERI L.
The successfulness of thein vitro micropropagation is achieved when the plant survives in the field soil without support of growth hormones and synthetic media by transplantation techniqueB. monnieri L. rooted plantlets from culture bottle were transferred to polythene bags containing eleven different sterile soil mixtures. Amongthem, maximum shoot length and root length were observed with plant grown in soil + VAM (10:2) and soil with vermicomposd (4:1) respectively. The percentage of survival was recorded as80-90% in the transplantation. Minimum shoot length (6.01±0.09 cm) and root length (4.29±0.12 cm) were recorded with plant grown in soil alone. The other soil mixtures induced moderate to minimum shoot and root growth of plants Thus, it has been reported that the growth of plants in the soils was controlled by various plant growth promoting substances like organic compounds. Previous workers carried out similar transplantation studies atin vitro conditions using various plants such as Bael tree (Aegle marmelos)
In cultivated field soil, the plant was grown well after45 d. Remarkably, the maximum shoot and root length was recorded in clay soil and clay + red soils respectively The percentage of the survival of the plant was 90%. Thus, it is clearly revealed that the growth of the plant not only influenced by nature and texture of the soil, but also the physico-chemical parameters of the soil greatly affected the growth and biochemical processes of the plants.(Fig no.5)
CONCLUSION
In the present study, a fruitful protocol was set up forB. monnieri through multiple shoot induction. This protocol can be exploited for commercial propagation and conservation of potential endangered medicinal plant resources. Comparison of growth pattern between various type of explants indicated that nodal segments exhibited higher growth rates (Table no.2) in term of shoot elongation occasional branching and multiplication when compared with shoot tips(Table no.2).Shoot tips explants mostly showed shoot elongation and multiplication was low. Multiple-shoot clumps showed elongation of the already formed shoot primordia and hence highest growth rate. Rooting was more or less uniform in all the explants types.
The comparison of field survival and growth of plants from different conditions are also varied. Percentage of survival on planting out was comparatively poor in plants transferred directly from in-vitro conditions. Explants culture on MS medium without cytokinins failed to produce shoots even 4 week. BAP in combination with 2,4-D and NAA markedly enhanced the percent regeneration, number of shoot and shoot length. Among all the cytokinins-auxin combinations tried, the maximum percent regeneration, number of shoots (90 %) and shoot length (4.95±0.9) per explant were obtained at IAA andBAP/2,4-Dnumber of shoots (95 %)shoot length (4.90±00).The role of auxin incorporated in the medium in combination with cytokinins for shoot multiplication has been reported in number of cases.
REFERENCES
1. Jarald E.D., Jarald S.E.(2007) test book of pharmacognosy & Phytochemistry.1st edition.CBSPublishers & distributors,New Delhi514-521.
2. Mishra S.P. (2009). plant tissue culture. Ane books pvt. Ltd. New Delhi.30-40.
3. Zimmerman J. L. (1993). Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423.
4. Tiwari Bhrigu Narayan, Khatri Pankaj et al (2010), tissue culture of endangered bael tree (aegle marmelos)-a review, j adv.sci.res. 1(2), 34-40.
5. Kumar U. (2002). methods in plant tissue culture.2nd edition.agrobis (India).66-87.1117.138-147.
6. Kalia A.N.(2005). test book of industrial pharmacognosy. 1st edition.CBS publishers & distributors.New Delhi.97-130.
7. Soundararajan T. and Karrunakaran C.M. (2001).Micropropagation of Bacopa monnieri through Protoplast”Asian Journal of Biotechnology,3 (2),135-152
8. Patil G.S., Shimp T.S.I., Deshpandey H.A, NarkhedeJ.D., Bhalsing S.R .(2009).Tissue culture studies on bacopa monnieri pennel–A Threatened medicinal herb.International Research Journal:I (1).
9. Vijayakumar M., Vijayakumar R. and StephenR.(2010)In vitro propagation of Bacopa monnieri L. - a multipurpose medicinal plant , Indian Journal of Science and Technology, (3)7.
10. George S., Geetha S.P., Balachandran I., Ravindran P.N. (2004).Micropropagation and in vitro conservation studies in Bacopa monnieri (L.) Pennell—The MemoryPlus Plant. In: Abstracts of the 26th Annual Meeting of the Plant Tissue Culture Association of India and National Symposium on Biotechnology for a Better Future. St. Aloysious College, Mangalore, India,15–17, 32.
11. Garai S, et al.(1996) Dammarane-type triterpenoid saponins from Bacopa monniera . Phytochemistry ;42:815.
12. Garai S, et al.(1996) Bacopasaponin D--a pseudojujubogenin glycoside from Bacopa monniera . Phytochemistry;43:447.16. Schulte K, et al. Components of medicinal plants. XXXVI. Nicotine and 3-formyl-4-hydroxy- 2H-pyran from Herpestis monniera . Phytochemistry 1972;11:2649.
13. Rastogi, S., R. Pal and D.K. Kulshreshtha(1993).Bacoside A3-a triterpenoidsaponins from Bacopa monnieri. Phytochemistry, 36(1): 133-137.
14. Chatterji N., Rastogi R.P. and Dhar M.L. (1963). Chemical examination of Bacopamonnieri Westtst.: Part I-isolation of chemical constituents. Indian J. Chem., 1: 212-215.
15. Jarald E.D., Jarald S.E.(2007) test book of pharmacognosy & Phytochemistry.1st edition.CBSPublishers & distributors,New Delhi514-521.
Reference ID: PHARMATUTOR-ART-1011
FIND OUT MORE ARTICLES AT OUR DATABASE









