About Authors:
Jigar Modi*1, Dr. Jayvadan Patel1, Dr. Hitesh chavda2
1Nootan Pharmacy College, S.P. Sahkar Vidhyadham,
Kamana Crossing, Visnagar-384 315,
Dist – Mehsana, Gujarat, India.
2Shri Sarvajanik Pharmacy College,
Nr. Arvind Baug, Mehsana, Gujarat, India
*jigo_farmacy@yahoo.com
ABSTRACT:
Generally, controlled release dosage forms used in many applications. Superporous hydrogel is also one of them. It has lot of advantages over conventional hydrogel. Superporous hydrogel having faster swelling due to interconnected highly porous structure. Such other properties like slippery property, their mechanical strength and better foaming process are advantageous over conventional hydrogel. Its unique mechanism for achieving gastric retention, a number of hurdles have to be overcome for any approach to be clinically useful. For example, intra-gastric floating systems require the presence of gastric juice to be effective, and this may not be the case in the fasted state. Mucoadhesive systems can easily lose their mucoadhesive properties by interaction with any materials soluble in gastric juice. In this review, types of superporous hydrogel, their preparation, their characterizations and applications are discussed.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1345
INTRODUCTION:
Hydrogel is a three dimensional network of polymer chains that are hydrophilic, sometimes found as a colloidal gel in which water is the dispersion medium. Hydrogels are highly absorbent (they can contain over 99.9% water) natural or synthetic polymers. Hydrogels also possess a degree of flexibility very similar to natural tissue, due to their significant water content. 1
Superporous hydrogel:
A superporous hydrogel is a three dimensional network of a hydrophilic polymer which absorbs a large amount of water in short time because of the presence of interconnected pores which having ideally range of 100 µm-600 µm. The size and number of the pores can be controlled by adjusting the type and amount of surfactant and gas forming agent during cross-linking polymerization. Even after drying, the pores in the SPHs remain connected to each other to form capillary channels.
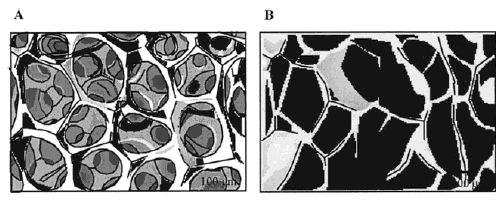
Figure 1: Presentation of superporous hydrogel (A) & conventional hydrogel (B) in the dried state.
No pores are observed in conventional hydrogels by SEM.
It should be noted that the SPHs have distinctly different properties compared to microporous and macroporous hydrogels. First, the pore sizes in the SPHs are several hundred micrometers and can increase up to the millimeter range; this is much larger than the pores in microporous or macroporous hydrogels. Second, in contrast to conventional microporous or macroporous hydrogels, which contain a relatively small fraction of empty spaces, the SPHs can easily accommodate gas cells more than several hundred percent of the volume of the starting monomer mixtures. Third, the pores in the SPHs remain connected even after drying, and this allows the dried hydrogels to swell extremely fast. 2
[adsense:468x15:2204050025]
Basic requirements for gastric retention of superporous hydrogel:
Based upon GI physiology, Superporous hydrogels must possess following properties in order to act as gastric retention device.
A. Initial size should be small enough for easy swallowing.
B. Swelling should be fast enough to overcome gastric emptying by IMMC.
C. Size of swollen hydrogel should be large enough to be retained in the stomach. D. Swollen hydrogel should be strong enough to withstand contraction pressure, abrasion and Shear forces in stomach (i.e. more than 50-70 cm water pressure).
E. To be applicable in humans as a drug delivery vehicle, a superporous hydrogel dosage form should be eliminated from the stomach after drugs are released. This can be obtained by either mechanical degradation to break the dosage form into small pieces or by chemical or enzymatic degradation by including biodegradable crosslinkers, like glycidyl acrylate modified albumin.
Principle of the gastric retention of superporous hydrogels or its composites
The superporous hydrogel having fast swelling property. Due to this property, gastric retention can be achieved by SPHs or its composites. A superporous hydrogel is filled into a capsule. So initial volume of this gastro-retentive dosage form is too small and easy to swallow. After oral administration, it swells rapidly in the gastric fluid to a large size, so that its emptying into the intestine is prevented. When the gastric contraction reaches the hydrogel, the gastric tissues slide over the hydrogel, because of its elastic and slippery property. When a drug is released from this dosage form, it slowly undergoes degradation in the stomach by either mechanical force or chemical/enzymatic hydrolysis of the polymer chains constituting the hydrogel. Eventually, the degraded superporous hydrogel dosage form is eliminated from the stomach. 3
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Various types/generation of superporous hydrogel
The facts that SPHs absorb water very fast even in large sizes makes them useful in the development of gastrointestinal platforms. The fully swollen SPHs, however, are mechanically very poor to meet the requirements for certain applications for which very high mechanical property (in their swollen state) is highly demanded. To distinguish SPHs with different properties, SPHs are divided into three different generations. The conventional (i.e., the first generation) SPHs is characterized by fast swelling, high swelling ratio and weak mechanical properties. On the other hand, the second-generation SPHs (SPH composites) are characterized by fast swelling, medium swelling ratio and improved mechanical properties. The third generation SPH hybrids possess elastic properties that can be highly useful in the development of gastrointestinal devices, as well as in other pharmaceutical and biomedical applications. 4
Different generations of superporous hydrogels are as follows.
First generation
These are first introduced by chen et al., for gastro-retentive drug delivery, also known as conventional superporous hydrogels (CSPH) with fast swelling kinetics and highly porous structure. The most commonly used monomers for synthesis of the first generation of SPHs are highly hydrophilic acrylamide, salts of acrylic acid and sulfopropyl acrylate. The dried SPHs are hard and brittle, but the hydrophilic nature of the polymer results in moisture induced plasticization of the rigid structures into soft and flexible structures. The dried SPHs swell fast to a large size, larger than a few hundred times of their own volume in the dried state. Due to extremely small fraction of the polymer in the swollen state, the swollen SPHs are sometimes difficult to handle without breaking. When the SPHs are dried, the porous structure become collapsed or shrunken due to the surface tension of water pulling the polymer chains together during the drying process. To avoid this problem, water inside SPHs is replaced with alcohol (e.g., ethanol). The low surface tension of alcohol prevents the porous structure from collapsing during drying. The CSPH are fragile against bending or tensile stresses. Their structures are easily broken apart even under very low pressures. The lack of desirable mechanical properties of the conventional SPHs triggered the development of the
second generation
SPH composites. 4 Second generation These are also known as superporous hydrogels composites (SPHC). These higher modulus hydrogels were introduced by chen et al., in 2000, as an improvement over conventional SPHs in terms of higher mechanical strength. A composite is a matrix of a continuous phase having a dispersed phase incorporated within. Composite structures are generally made to attain certain properties, which cannot otherwise be achieved by each matrix alone. For making SPH composites, a matrix-swelling additive or a composite agent is utilized. A composite agent used in SPH composites is a cross-linked water-absorbent hydrophilic polymer that can absorb the solution of monomer, cross-linker, initiator and remaining components of the SPH synthesis. Upon polymerization, the composite agent serves as the local point of physical cross-linking (or entanglement) of the formed polymer chains. During the polymerization process, each composite agent particle acts as an isolated individual reactor in which cross-linking polymerization occurs. As the cross-linking polymerization proceeds throughout the solution, individual swollen composite agent particles are connected together through polymer chains connecting them. The presence of composite agent in SPH composites results in improved mechanical properties over conventional (i.e., the first generation) SPH, but the SPH composites are still brittle and thus break into pieces upon application of stresses. This modification over conventional SPHs resembles modification of superabsorbent polymers through surface cross-linking. Overall, this type of modification results in a higher modulus polymer network in the swollen state, which is susceptible to failure under the brittle fracture mechanism. For many years, these second generations of SPHs have been an attractive research tool for peroral and intestinal drug delivery applications. The most widely used composite agents are crosslinked sodium carboxymethylcellulose (ac-di-sol), crosslinked sodium starch glycolate (primojel) and crosslinked polyvinylpyrrolidone (crosspovidone). Polyvinyl alcohol, carbopols are also used to improve the mechanical strength of SPHs. Though, this modification leads to polymeric networks with improved mechanical strength in swollen state but still these are prone to breakdown under high tensile stress4. Chavda et al., formulated newer approach on SPH composite based bioadhesive system. The proposed bioadhesive, mechanically stable as well as floating drug-delivery system based on superporous hydrogel composite containing carbopol 934P as a composite material is promising for stomach specific delivery of ranitidine hydrochloride.5 Wang et al., synthesized SPH composite beads based on chitosan-g-poly (acrylic acid) /vermiculite and sodium alginate for diclofenac controlled release. It was prepared using chitosan-g-poly (acrylic acid)/vermiculite composite and sodium alginate by Ca2+ as the crosslinking agent. The release rate of the drug from the composite hydrogel beads is remarkably slowed down, which indicated that incorporating vermiculite into the composite hydrogel beads can improve the burst release effect of the drug.6
Third generation
Further advancement in mechanical strength leads to third generation SPHs which includes super- porous hydrogels interpenetrating networks (SPH-IPNs) and superporous hydrogel hybrid (SPHH). A second polymeric network is incorporated into SPH frame to form interpenetrating network structure in case of SPH-IPNs. A water soluble hybrid agent is introduced in SPHs. The hybrid agent evenly diffuses and dissolves into polymer solution leading to formation of integrated semi interpenetrating network which upon treatment of hybrid agent yields integrated IPNs structure. They can withstand various types of stresses like compression, bending and twisting etc. Various hybrid agents have been used and specific treatment has been applied to get integrated IPNs hydrogels, e.g. natural hydrocolloids like sodium alginate, chitosan, sodium carboxymethylcellulose, and pectin and synthetic water soluble polyvinyl alcohol. Natural hydrocolloids show ionotropic gelation via treatment with metal ion like calcium, iron etc. (e.g. sodium alginate with ca+2 ions, chitosan with phosphates). Ethylenebisacrylamide is used as a thermally resistant chemical crosslinker. Cerium ammonium nitrate is used to prepare grafted SPHHs. Stronger SPHHs can be prepared by replacing the diacrylate crosslinker with a trifunctional acrylate. The SPHHs having lower salt sensitivity can be prepared using a quaternary ammonium salt (diallyldimethyl ammonium chloride) as a secondary monomer.4 Gupta et al., developed gastro-retentive drug delivery system based on SPH. The aim of this work was to synthesize superporous hydrogels of rosiglitazone using chitosan and to study its swelling behavior for application as a gastro-retentive drug delivery system.7 Lichen et al., studied about polymer integrity related absorption mechanism of SPH containing interpenetrating polymer networks for oral delivery of insulin. SPH containing poly (acrylic acid-co-acrylamide)/o-carboxymethyl chitosan interpenetrating polymer network (SPH-IPN) was evaluated as the oral delivery vehicle for insulin, emphasizing on the effect of polymer integrity on insulin absorption mechanisms. The integral SPH-IPN and powdered SPH-IPN exhibited potent and equivalent in vitro enzymatic inhibition capacities, which were attributed to both enzyme incorporation and Ca2+ deprivation. Results were further confirmed by in vivo assessment in that oral administration of insulin-loaded integral SPH-IPN yielded notable insulin absorption and hypoglycemic effect, while powdered SPH-IPN was ineffective.8
Miscellaneous SPHs:
Development of SPHs with mechanical properties identical to that of SPHC has been attempted applying different approaches, including acidification (using HCl), impregnation (using diallyldimethyl ammonium chloride or cationic polyethyleneimine or cationic resin of polyamidoamineepichlorohydrin), rubberization (adding rubber emulsions), surface crosslinking (using glycerin), ionotropic gelation (using synthetic polymers other than hydrocolloids; like polyvinyl acetate), bulk crosslinking (using higher concentration of a chemical crosslinker) thermogelation (using ovalbumine protein, egg white) and ionotropic gelation (using ion-complexable co-monomers; e.g. acrylic acid).4 Ranjha et al., synthesized hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. This hydrogel is formed due to electrostatic interaction between cationic groups in chitosan and anionic groups in acrylic acid.9
General preparation of superporous hydrogel:
Synthesis of superporous hydrogel is same to the synthesis of ordinary hydrogels but the only difference is that a foaming agent is added to prepare superporous hydrogels. The timing of the polymerization has to be matched with the timing of foam formation. If the kinetics of the two processes is not matched, then superporous hydrogels with interconnected pores will not be formed. The important step of this process is to use acid to control the polymerization kinetics. Addition of NaHCO3 leads to foam formation as well as rise in pH, which accelerates the polymerization process. After the addition of NaHCO3, polymerization becomes complete within a few minutes. The pH of the monomer mixture is low because of the addition of acid, and this makes polymerization very slow. The addition of NaHCO3 results in foaming and at the same time the pH of the solution rises. The pH increase accelerates the polymerization process, which is completed before the foam subsides. This results in formation of superporous hydrogel. 4
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Methods for preparation of superporous hydrogels:
Following methods are useful in the preparation of superporous hydrogel.
1) Porosigen technique
Porous hydrogels can be made by preparing the hydrogels in the presence of dispersed water-soluble porosigen, which can be removed later by washing with water to leave an inter-connected meshwork (i.e., porous hydrogels). Examples of effective porosigens are micronized sucrose, lactose, dextrin, sodium chloride, and polyethylene glycol.
2) Phase separation technique In solution polymerization, monomers are usually mixed in diluent that is good for both monomers and polymers. If, however, the diluent is a nonsolvent for the polymer formed (e.g., PHEMA in water), the solubility of the polymers dramatically decreases as the polymerization proceeds. This results in phase separation of the polymer rich monomer phase into droplets, which then join together to form a network ?lled with large spaces (i.e., heterogeneous, porous hydrogels) by the end of the polymerization process. This process is called heterogeneous solution polymerization. The pore sizes of macroporous hydrogels prepared by phase separation are typically only a few micrometers. In addition, the overall porosity is very low, and this implies that the pores are not well interconnected. The major limitation of the phase separation method is that only very limited types of porous hydrogels can be prepared. In addition, there is not much control over the porosity of the gels when prepared by phase separation.
3) Cross linking technique
Individual hydrogel particles can be crosslinked to form crosslinked aggregates. Pores are formed between the hydrogel particles. Such aggregate macrostructures were prepared by initially mixing the hydrogel particles (in the range of a few hundred micrometers) with a solution of a crosslinking agent, water, and hydrophilic organic solvent such as isopropanol. Pores in such structures are present between hydrogel particles, and the size of pores is much smaller than the size of particles. This approach is limited to the absorbent particles that have chemically active functional groups on the surface.
4) Gas blowing (or foaming) technique
Hydrogels can be prepared in the presence of gas bubbles. In this technique the monomers are polymerized or water-soluble polymer chains are crosslinked around gas bubbles generated by a blowing agent. The gas blowing technology has been widely used in the preparation of plastic foams from materials such as polyurethanes, rubber, and poly (vinyl chloride). The key ingredient in the foaming process is a blowing agent (or foaming agent), which is de?ned as any substance or combination of substances capable of producing cellular structure within a polymer matrix. Foaming agents are classified as; (a) physical foaming agents that expand when pressure is released (e.g., nitrogen and carbon dioxide) and (b) chemical foaming agents that decompose or react to form a gas (e.g., sodium bicarbonate in the presence of acid). Recently, the gas blowing technique was used in laboratory to prepare SPHs. Because this technique used is for the preparation of SPHs, they were also called “hydrogel foams.” In the synthesis of SPHs by the gas blowing technique, foaming and polymerization have to occur simultaneously. For this reason, it is important to control the timing for foaming and polymerization. In the study mentioned above, inorganic carbonates, such as sodium carbonate and sodium bicarbonate were used as the foaming agent. These inorganic carbonates have long been used safely as a gas-forming ingredient in effervescent tablets for antacids. They are safe, cheap, and easy to use. 10, 11
Ingredients used in preparation of superporous hydrogel:
Monomer: Acrylic acid, acrylamide, (meth) acrylic acid, salts of (meth) acrylic acid, esters of (meth) acrylic acid, amides of (meth) acrylic acid, methacrylamide, 2-hydroxyethyl methacrylate, n-isopropyl acrylamide, n-vinyl pyrollidinone etc. Crosslinking agent: N,N’-methylenebisacrylamide (bis) is used most widely in blowing Technique. Glutarldehyde (chemical crosslinker), metal ions like calcium, iron and phosphorus are used in ionotropic crosslinking of hydrocolloids. Such others are ethylene glycol di(meth)acrylate, piperazine diacrylamide, epichlorohydrin etc.
Foam stabilizer: Pluronic f127, pluronic p105, silwet l7605, span, tween etc. Polymerization initiator pair: APS/TEMED (ammonium persulfate/ N,N,N,N-tetramethylethylene- diamine, potassium persulphate/Sodium metabisulfite, APS/Sodium metabisulfite, azo-initiator (V545) etc.
Foaming agent: Sodium bicarbonate
Composite agent (superdisintegrant): Various superdisintegrants like crosslinked sodium carboxy- methylcellulose (ac-di-sol), crosslinked sodium starch glycolate (primojel) and crosslinked polyvinyl- pyrrolidone (crospovidone) are mostly used. Hybrid agent: natural polymers like sodium alginate, sodium carboxymethylcellulose, chitosan based on ionotropic gelation and some synthetic polymers like polyvinyl alcohol based on cryogelation. 4, 10
Characterization of superporous hydrogel
Swelling studies
Swelling time Swelling time was calculated by immersing the hydrogels in deionized water as well as 0.1N HCl and calculating the time required to attain equilibration in swelling. Swelling ratio The dried SPH was allowed to hydrate in excess of deionized distilled water at room temperature. The weight of hydrating sample was measured at time intervals, after excess water was removed by gentle blotting. The swelling ratio was defined as
Qs = Ws – Wd X 100
Wd
Where, Ws is the weight of the swollen superporous hydrogel and Wd is the weight of the dried superporous hydrogel.12, 13
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Density measurement
The solvent displacement method was used. Dried SPHC was used for density measurement, which actually shows the apparent density of the SPHC. Piece of SPHC was taken and weighed in order to determine the mass of piece. A piece of the polymer was immersed in a predetermined volume of hexane in a graduated cylinder, and the increase in the hexane volume was measured as the volume of the polymer. The density was calculated from equation
Density = MSPHC/VSPHC
Where, VSPHC is the volume of solvent displaced by SPHC and MSPHC is the mass of the SPHC.13-14
Porosity measurement
Dried SPHC was immersed in hexane over night and weighed after excess hexane on the surface was blotted. The porosity was calculated from equation
Porosity = VP/VT
Where, VP (= VT – VSPHC) is the pore volume of SPHC and VT is the total volume of the SPHC. Total volume of SPHC can be measured from its dimensions, as it is cylindrical in shape.14
Mechanical properties
The penetration pressure of the SPHC was measured using a bench comparator as described by chen et al. with modifications. The fully swollen hydrogel was put longitudinally under the lower touch and weights were successively applied to the upper touch until the polymer completely fractured. The compressive force could be read from the gauge, and the penetration pressure could be calculated from equation
PP = Fu/S
Where, PP is the penetration pressure, Fu is the ultimate compressive force at complete breakage of the polymer and S is the area of the lower touch.13
Gastric simulator
Generally the pores inside the SPH vary from 100 to 1000 µm in size. Under homogeneous loading, pores of different sizes resist deformation differently. The SPH mass will break apart from its weakest point, which cannot be monitored by using regular mechanical testers. A gastric simulator, based on the water hammer theory, utilizes a controlled amount of different types of stresses on objects immersed in the testing fluid to simulate the forces that a sample might receive upon ingestion in the body. The stress concentrated on the weakest part of the SPH body will lead to the formation of craze, crack and finally rupturing of the whole platform. The simulator measures the amount of energy absorbed by the sample until it fails under certain stresses. The gastric simulator is valuable in screening formulations and in designing SPH-based gastro-retentive platforms.15
Measurement of gelation kinetics
As the polymerization reaction proceeds, the viscosity continuously increases until the full network structure (gel structure) is formed. The gelation time is defined as a time period for gel formation. It is measured by a simple tilting method after adjustment of pH to 5.0 with acetic acid. It is determined by the duration of time taken by the reactant mixture to become viscous and henceforth the viscous solution no longer falls down in the tilted tube position.16
Water retention
The following equation is used to determine the water retention capacity (Wrt) as a function of time,
Wrt = ( Wp – Wd ) / ( Ws – Wd )
Where, Wd is the weight of the dried hydrogel, Ws is the weight of the fully swollen hydrogel, and Wp is the weight of the hydrogel at various exposure times.
For determination of the water-retention capacity of the hydrogels as a function of the time of exposure at 37 0C, the water loss of the fully swollen polymer at timed intervals was determined by gravimetry.11
Drug loading
The method of soaking or equilibration is used for drug loading. In this method the amount of buffer necessary for complete swelling of superporous hydrogel is determined. Thereafter the drug solution in the determined amount of buffer which is required for complete swelling is prepared. Subsequently, superporous hydrogel is placed in the drug solution and left until all the drug solution is sucked up. Then the completely swollen superporous hydrogel loaded with the drug is placed in an oven at 30 0C for drying overnight.11
Safety/toxicity or in-vivo Study
The safety and non-toxicity of synthetic superporous hydrogels must be demonstrated before these delivery systems can be pharmaceutically acceptable. Swine emesis model study has been used to investigate the safety of novel gastro-retentive SPH platforms.12, 15
Floating behaviour of SPH-DDS
The SPH-DDS were placed in a 100 mL beaker containing 0.1 N HCl. The time required for SPH-DDS to rise to the surface and float was taken as the floating lag time. Total time period for which SPH-DDS remains. Buoyant was considered as a total floating time.13
Morphological examination
The morphology of porous structures was examined with a scanning electron microscope (SEM). Superporous hydrogel composites were cut to expose their inner structure, coated with a thin layer of palladium gold alloy in hummer 1 Sputter Coater, and imaged in an SEM.16
Applications of superporous hydrogel
Sustained drug delivery
These systems can remain in the stomach for long period of time and thereby can release the drug over a prolonged period. The problem of short gastric residence time encountered with an oral controlled release formulation can be overcome with these systems. These systems have a bulk density of less than one as a result of which they can float on the gastric contents. These systems are relatively large in size so that they cannot pass through pyloric opening.
Site-specific drug delivery
These systems are particularly useful for drugs that are specifically absorbed from stomach or the proximal part of the small intestine e.g. riboflavin and furosemide. A bilayer floating capsule was developed for local delivery of misoprostol, which is a synthetic analogue of prostaglandin E1 used in gastric ulcers caused by use of non-steroidal anti-inflammatory drugs. By targeting slow delivery of misoprostol to the stomach, desired therapeutic levels could be achieved and drug waste could be reduced.
Gastro-retentive dosage forms
Dry blending and direct compression is used to make gastro retentive tablets. The SPH particles of acrylic acid/sulfopropyl acrylate copolymers are mixed with gelatin and tannic acid, and then tableted by direct compression. Formation of hydrogen bond between gelatin and tannic acid, as well as the carboxyl groups on the polymeric carrier, produce an integrated matrix, which is shown to be stable after swelling. The gastro retentive tablet can swellup to 22 times its own volume within a 40 min. period maintaining its original shape.Chenet al. studied about gastric retention properties of superporous hydrogel composites. The gastric retention property of the prepared SPH composites was tested in dogs both in fasted and fed conditions. The SPH composites were placed in a hard gelatin capsule (size 000) for oral administration.
Peroral peptide delivery systems
The feasibility of using CSPHs and SPHCs for peroral peptide delivery has been investigated. These systems are designed to swell in the intestine with the SPHs physically adhering to the gut wall and delivering the incorporated peptide directly to the site. The carboxyl-carrying SPHs can potentially induce calcium extraction, presumably causing the tight junctions of the gut wall to open and deactivating the harmful gut enzymes. After peptide delivery and absorption across the gut wall, the SPHs become over-hydrated and are broken apart by the peristaltic forces of the gut. The proper selection of the type and thickness of enteric coating will potentially help to target this dosage form to any specific site in the small intestine or to the colon.
Fast-dissolving tablets
Fast-dissolving tablets are orally administered without the need for water and swallowing which is beneficial especially to children and the elderly. The methods used to prepare fast-melting tablets are freeze-drying, sublimation and direct compression. The first two methods make tablets that dissolve within 5–15 seconds, but the technology is expensive and tablets prepared are not strong mechanically. Another method of preparation of fast-dissolving tablets by the direct compression method is the addition of fine particles of SPHs to the granulation or powder formulation. The SPHs microparticles within the tablet core accelerate water absorption by an increased wicking mechanism. Tablets prepared by direct compression with the use of SPHs microparticles disintegrate in less than 10 sec.
Development of diet aid
Diet soft drinks, meal replacement shakes, diet drugs and even surgical methods have been used to lose weight. The SPHs can theoretically occupy a significant portion of the stomach space due to their rapid and extensive swelling, leaving less space for food, and thereby suppressing appetite. This type of system can help to lose weight in obese people. Maintaining the integrity and volume of the swollen SPHs for a substantial period of time is the major challenge in the use of SPHs as a weight loss aid.
Chemoembolization and occlusion devices
Chemoembolization is a combined method of embolization and chemotherapy. Embolization has been used for the treatment of cancer by restricting the oxygen supply to the growing tumours. This method can be combined with chemotherapeutic agents to achieve local delivery and low systemic toxicity. A chemotherapeutic agent and an anti-angiogenic agent can be loaded into SPHs for chemoembolization therapy. The strong SPHs are better candidates for this application because they fit better in the blood vessels and provide better blocking.
Development of occlusion devices for aneurysum treatment
SPHs can also be used to produce biomedical devices for the treatment of aneurysms. An equivalent SPHs is prepared in smaller size after determining the size and shape of an aneurysm site. When a superporous hydrogel is positioned at the aneurysm site, it swells quickly to occupy the space and make the blood clot. Deposition of superporous hydrogels can result in up to 95% aneurysm occlusion without any evidence of parent artery compromise and inflammatory response. A new occlusion device prepared by combination of superporous hydrogel and platinum coils, called as, hydrocoil, is currently under development by Micro-Vention, Inc, in Aliso Viejo, California.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Other applications:
SPHs can also be used in industries other than pharmaceutical and biomedical, where rapid and extensive swelling in an aqueous medium is required. The use of SPHs is beneficial to hygiene, agriculture, horticulture, pet, toy and many other industries in their products. The immediate swelling of SPHs can be enjoyed by the children and they can learn the associated science and knowledge as it is shown with the super absorbent polymers. The SPHs can be coloured and may have decorative applications. SPHs may be a suitable substitute for silica gel as they quickly absorb moisture from the surrounding environment. The high swelling pressure of SPHs can potentially be used to trigger an alarm system upon the penetration of water.12, 15 Modifications of poly (2-hydroxyethyl methacrylate) with cholesterol and the introduction of large pores have been developed to create highly superporous hydrogels that promote cell-surface interactions and that can serve as a permissive scaffold for spinal cord injury treatment. Highly superporous cholesterol-modified poly (2-hydroxyethyl methacrylate) scaffolds have been prepared by the bulk radical copolymerization of 2-hydroxyethyl methacrylate, cholesterol methacrylate and ethylene dimethacrylate crosslinking agent in the presence of ammonium oxalate crystals to establish interconnected pores in the scaffold.17
Other general uses of superporous hydrogel are as follows:
• Environmentally sensitive hydrogels which are also known as 'smart gels' or 'intelligent gels'. These hydrogels have the ability to sense changes of pH, temperature, or the concentration of metabolite and release their load as result of such a change.
• As sustained-release drug delivery systems.
• Provide absorption, desloughing and debriding of necrotic and fibrotic tissue.
• Hydrogels that are responsive to specific molecules, such as glucose or antigens, can be used as biosensors, as well as in drug delivery.
• Used in disposable diapers where they absorb urine, or in sanitary napkins.
• Contact lenses (silicone hydrogels, polyacrylamides).
• Water gel explosives.
• Rectal drug delivery and diagnosis.
Other, less common uses include;
• Breast implants.
• Now used in glue to seal up the vagina if bleeding.
• Granules for holding soil moisture in arid areas.
• Dressings for healing of burn or other hard-to-heal wounds. Wound gels are excellent for helping to create or maintain a moist environment.
• Reservoirs in topical drug delivery; particularly ionic drugs, delivered by iontophoresis (see ion exchange resin) common ingredients are e.g. polyvinyl alcohol, sodium polyacrylate, acrylate polymers and copolymers with an abundance of hydrophilic groups. Natural hydrogel materials are being investigated for tissue engineering; these materials include agarose, methylcellulose, hyaluronan, and other naturally derived polymers.18, 19
CONCLUSION:
Superporous hydrogels are promising tools to achieve prolonged residence time in stomach for many drugs. Superporous hydrogels are porous crosslinked structures with the ability of absorbing aqueous fluids few hundred times their own weight. These systems should swell in stomach; maintain their integrity due to better mechanical strength. Superporous hydrogels, their composites and superporous hydrogel hybrids holds a lot of potential for pharmaceutical and biomedical in future, because of their good elastic property, superb swelling and mechanical strength.
ABBREVIATION:
SPHs- Superporous hydrogel
SPHC- Superporous hydrogel composite
SPHH- Superporous hydrogel hybrid
SPH-IPNs- Superporous hydrogels interpenetrating networks
PHEMA- Poly(2-hydroxyethyl methacrylate)
APS- ammonium persulfate
TEMED- N,N,N,N-tetramethylethylenediamine
SPH-DDS- Superporous hydrogel drug delivery system
REFERENCES:
1. iupac.org/goldbook/X06700.pdf
2. Mahdavinia GR, Mousavi SB, Karimi F, Marandi GB, Garabaghi H, Shahabvand S, Synthesis of porous poly (acrylamide) hydrogels using calcium carbonate and its application for slow release of potassium nitrate. Express Polymer Letters, 2009, 3(5), 279–285.
4. Hossein Omidian, Rocca JG, Park K, Review advances in superporous hydrogels. Journal of Controlled Release, 2005, 3 –12.
3. Jun Chen, William E. Blevins, Haesun Park, Kinam Park, Gastric retention properties of superporous hydrogel composites. Journal of Controlled Release, 2000, 64, 39–51.
5. Chavda HV, Patel CN, A newer formulation approach: superporous hydrogel composite-based bioadhesive drug-delivery system. Asian Journal of Pharmaceutical Sciences, 2010, 239-250.
6. Wang Q, Xie X, Zhang X, Zhang J, Wang A, Preparation and swelling properties of pH sensitive composite hydrogel beads based on chitosan-g-poly (acrylic acid)/vermiculite and sodium alginate for diclofenac controlled release, Int. J. Boil Macromol., January 2010.
7. Gupta NV, HG Shivakumar, Development of a gastro-retentive drug delivery system based on superporous hydrogel. Tropical Journal of Pharmaceutical Research, June 2010, 9(3), 257-264.
8. Lichen Y, Likun F, Fuying C, Cui T, Chunhua Y, Polymer integrity related absorption mechanism of superporous hydrogel containing interpenetrating polymer networks for oral delivery of insulin. Biomaterials., January 2010.
9. Bhanja SB, Ellaiah P, Chandan M, Murthy KVR, Panigrahi B, Padhy SK, Hybrid pH sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J. Mater. Sci. Mater. Med., October 2010.
10. Nagpal M, Singh SK, Mishra D, Superporous hydrogels as gastro-retentive devices. Acta Pharmaceutica Sciencia, 2011, 7 – 24.
11. Patel PK, Mistry SN, Patel GJ, Dr. Bharadia PD, Pandya VD, Modi DA, Recent in controlled drug delivery system: superporous hydrogels. IJPI’s Journal of Pharmaceutics and Cosmetology, 2011, 53-65.
12. Park k, Superporous Hydrogels for Pharmaceutical & Other Applications, Drug Delivery Technology, July-August 2002, 2(5), 38-44.
13. Chavda HV, Patel CN, A newer formulation approach: superporous hydrogel composite-based bioadhesive drug-delivery system. Asian Journal of Pharmaceutical Sciences, 2010, 239-250.
14. Chavda HV, Patel CN, Effect of crosslinker concentration on characteristics of superporous hydrogel. International Journal of Pharmaceutical Investigation, January 2011, 11-21.
15. Omidian H, Park K, Rocca JG, Recent developments in superporous hydrogels. Journal of Pharmacy and Pharmacology, 2007, 317–327.
16. Gupta NV, HG Shivakumar, pH- sensitive superporous hydrogel composed of methacrylic acid and acrylamide: preparation and properties. Acta Pharmaceutica Science, 2010, 52, 239-246.
17. Kubinova S, Horak D, Hejcl A, Plichta Z, Kotek J, Sykova E, Highly superporous cholesterol modified poly(2-hydroxyethyl methacrylate) scaffolds for spinal cord injury repair. J. Biomed. Mater. Res., September 2011.
18. Kuang J, Kun YY, Kang MH, Polysaccharide based superporous hydrogels with fast swelling and superabsorbent properties. J. Mater. Sci. Mater. Med., August 2010.
19. Cetin D, Kahraman AS, Menemse G, Novel scaffolds based on poly (2-hydroxyethyl methacrylate) superporous hydrogels for bone tissue engineering. J. Biomater. Sci. Polymed., July 2010.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









