
Vipra Adlakha, Sunita Nirban, Sunil Kumar
Lord Shiva College of Pharmacy,
Sirsa,
Haryana
Abstract
Transdermal drug technology specialists are continuing to search for new methods that can effectively and painlessly deliver larger molecules in therapeutic quantities to overcome the difficulties associated with the oral route, namely poor bioavailability due to hepatic metabolism (first pass) and the tendency to produce rapid blood level spikes (both high and low). As all the drugs do not meet the physicochemical properties required for transdermal drug delivery. So, these techniques are required. Although addition of penetration enhancers increases permeation of drugs but that is not sufficient to ensure delivery of therapeutically effective concentration of drug therefore, several new active rate controlled TDDS technologies (drug and vehicle drug selection, vesicles and particles, Horney layer modified, Horney layer bypassed, Electrically driven and some other techniques) are being employed. This review article covers most of the new active transport technologies involved in enhancing the transdermal permeation into an effective DDS.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1109
Transdermal drug delivery system also known popularly as „patches? are essentially a collection of technologies designed to deliver therapeutically effective amount of drug to the body across a patient?s skin.(1).
Over the last 2-3 decades, the skin has become an important route for the delivery of drugs for topical, regional or systemic action (2). Thus, transdermal route has vied with oral treatment as the most successful innovative research area in the drug delivery (3). In particular, it is used when there is a significant first-pass effect of the liver that can prematurely metabolize drugs. Transdermal delivery also has advantages over hypodermic injections, which are painful, generate dangerous medical waste and pose the risk of disease transmission by needle re-use, especially in developing countries (4). In addition, transdermal systems are non-invasive and can be self-administered.
Vaccine delivery via the skin is even more attractive because it targets the potent epidermal Langerhans and dermal dendrite cells that may generate a strong immune response at much lower doses than deeper injection (5). In a world where needle reuse kills at least 1.3 million people per year from hepatitis B and AIDS (4), needle-free, patch-based vaccination could have large impact. With current delivery methods, successful transdermal drugs have molecular masses that are only up to a few hundred Daltons, exhibit octanol-water partition coefficients that heavily favour lipids and require doses of milligrams per day or less (6-9). It has been difficult to exploit the transdermal route to deliver hydrophilic drugs; the transdermal deliver of peptides and macromolecules, ionic drugs including new genetic treatment employing DNA or small-interfering RNA (10-11).The major barrier within the skin is the stratum corneum, the top layer of the epidermis. The stratum corneum consists of keratinized, flattened remnants of once actively dividing epidermal cell. Hygroscopic, but impermeable to water, it behaves as a tough, flexible membrane. The intercellular space is rich in lipids. This means that considerable effort has to be expended on the appropriate design of a formulation or a device to deliver enough of the medicine such that there is sufficient drug present at its site of action. (12). To overcome the disadvantages of transdermal drug delivery and to exploit it for all drugs different approaches have been applied which are classified as follows:
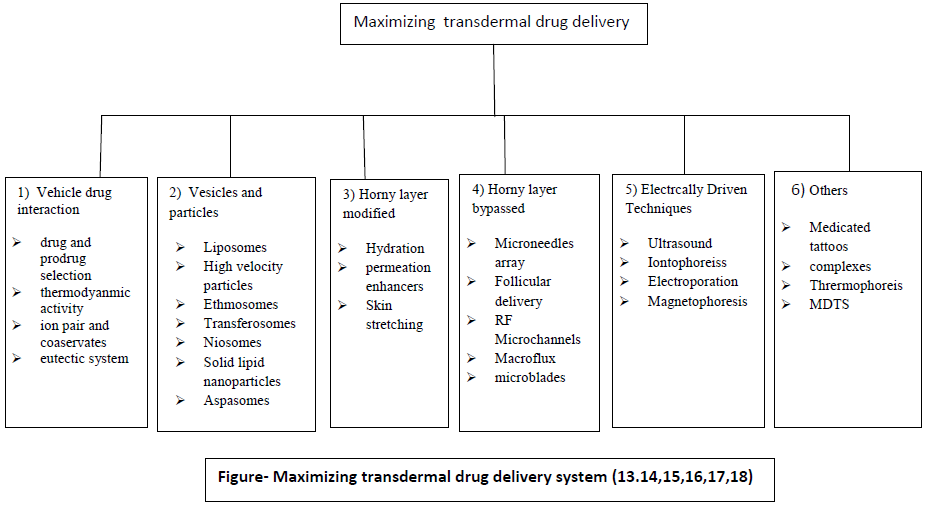
1) Vehicle drug interaction
Drug and prodrug selection
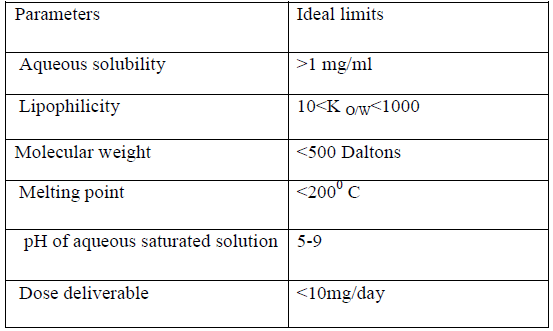
Drug should be selected in such way that it fits in the criteria of transdermal delivery as given in table 1(19-24).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Prodrug strategy generally involves addition of a pro-moiety to increase partition coefficient and solubility to increase the partition coefficient and solubility to increase the transport of drug in the stratum corneum (25). Upon reaching the viable epidermis, esterase releases the parent drug by hydrolysis. For e.g. the intrinsic poor permeability of the very polar 6-mercaptopurine was increased up to 240 times using S-6- acyloxymethyl and dialkylaminomethyl promoieties (26). In some cases aqueous solubility of prodrug is also lower than parent drug as in case of naltrexone base (27). Also prodrug approach may be used to avoid skin irritation (28).
Limitations of prodrug approach
• Prodrug design is complex.
• Applicable to a small molecule drugs
• It needs to gain US Food and Drug Administration approval of the prodrug as a new entity (28).
Thermodynamic activity
The maximum skin penetration rate is obtained when a drug is at higher thermodynamic activities in case of supersaturated solution (29). This increases the concentration gradient (C0-Ci) in the Fick?s law and thus forces the active ingredient out of formulation and in to across the stratum corneum (30).several methods can be used to produce supersaturated systems:
• Heating and subsequent cooling
• Removal of solvent
• Reaction of two or more solutes to produce a compound which is lesws soluble
• Addition of a substance to a solution that reduces the solubility of the solute(30)
In addition if water is imbibed from the skin in to the vehicle and acts as an antisolvent, thermodynamic activity of solvent would increase(29). The major advantage of this technique is its non interference with the barrier properties of the stratum corneum. However supersaturated system are thermodynamically unstable. Some polymers like polyvinylpyrrolidone. Polyethylene glycol. Eudragit, Polymethacrylates, dextran derivatives, cellulose esters act as an nucleating agents and can control the crystallisation process and enhance permeation of a number of a drugs (31-35).
Ion pair and coacervates
Charged drug molecules do not readily partition into or permeate through human skin. Formation of lipophilic ion pairs has been investigated to increase stratum corneum penetration of charged species. This strategy involves adding an oppositely charged species to the charged drug, forming an ion-pair in which the charges are neutralised so that the complex can partition into and permeate through the stratum corneum. The ion-pair then dissociates in the aqueous viable epidermis releasing the parent charged drug, which can diffuse within the epidermal and dermal tissues (36).
A coacervate is a tiny spherical droplet of assorted organic molecules (specifically, lipid molecules) which is held together by hydrophobic forces from a surrounding liquid (37). The coacervate partitions into stratum corneum, where it behaves as ion pairs, diffusing, dissociating and passing into viable tissues (38).
Eutectic system
The melting points of a drug influences solubility and hence skin penetration. According to regular solution theory, the lower the melting point, the greater the solubility of a material in a given solvent, including skin lipids. The melting point of a drug delivery system can be lowered by formation of a eutectic mixture: a mixture of two components which, at a certain ratio, inhibit the crystalline process of each other, such that the melting point of the two components in the mixture is less than that of each component alone (36,38,39). A number of eutectic systems containing a penetration enhancer as the second components have been reported, for example: Ibuprofen with terpenes, and methyl nicotinate, propranolol with fatty acids, and lignocaine with menthol. In all cases, the melting point of the drug was depressed to around or below skin temperature thereby enhancing drug solubility (40-43).
2) Vesicles and particles
Liposomes
Liposomes are colloidal particles formed as concentric biomolecular layers that are capable of encapsulating drugs. These lipid molecules are usually phospholipids with or without some additives.10 Cholesterol may be included to improve bilayer characteristics of liposomes; increasing microviscosity of the bilayer, reducing permeability of the membrane to water soluble molecules, stabilizing the membrane and increasing rigidity of the vesicles (44).
Five potential mechanisms of action of these Liposomes were assessed
• A free drug process-drug releases from vesicle and independently permeates skin
• Enhancement due to release of lipids from vesicles and interactions with skin lipids
• Improved drug uptake by skin
• That different entrapment efficiencies of the liposomes controlled drug input
• Penetration of stratum corneum by intact liposomes (3).
The composition of lipid and method of preparation markedly affect their transport across the skin. Deposition of liposomes prepared from lipids with composition similar to stratum corneum molecules in the deeper layer is better than that liposomes prepared from phospholipids. Similarly liposomes made of ceramides are more effective in penetrating the intact cutis than simple phospholipids vesicles (45).
High velocity particles
Also called as needle less injection. Transdermal delivery is achieved by firing the liquid or solid particles at supersonic speeds through the outer layers of the skin using a suitable energy source (46). The claimed advantages of the system include
• Pain free delivery particles are too small to trigger pain receptors in skin
• Improved efficacy and bioavailability
• Targeting to a specific tissue, such as a vaccine delivered to epidermal cells
• Sustained release, or fast release
• Accurate dosing
• Overcome needle phobia
• Safety - the device avoids skin damage or infection from needles or splash back of body fluids particularly important for HIV and hepatitis B virus (47).
Some of the needle free injector under development are:
Intraject: one of the prefilled disposable injectors intraject, under development, to use the nitrogen propelled device, which has a blank drug capsule. The patient snaps off the tip, tears off the safety end and plenus the nozzle against the skin pressurized gas and pushes the liquid formulation through a narrow orifice in to the skin.
Implaject: It first pushes a tiny, potential “pioneer tip, through the skin ahead of the drug. The tip pierces the tissue, creating a channel through which the therapeutic agent follows immediately.
Jet syringe: the jet syringe which can deliver up to 0.5 ml; can be configured with a an adjustable dose fill able ampoules or proprietary prefilled glass ampoule for fixed dose applications.
Iject: it is light weight, hand held liquid NFI. It can deliver 0.1 to1.0 mol subcutaneously or intramuscularly.
Miniject: it utilises a glass cartilage to accommodate for long term drug storage and stability, a polycarbonate syringe to accommodate for wide range of pressure profile and proprietary multiphase energy system, that can deliver specific pressure profile to ensure that the entire drug is deliver comfortably. It can target specific tissue layer.
Crossject: it comprises three modules. The gas generator contains the chemical energy source and is triggered by the impact of a syringe, the drug container and the third module nozzle, of polycarbonate with one or more orifices depending on the quantity of the formulation (48).
Powerject devices: the powerject system of transdermal delivery of powdered drug involves the propulsion of solid drug particle in to skin by means of a high speed gas flow. This method is painless causing no bleeding and damage to the skin (49).
Ethmosomes
These are liposomes with with a high alcohol content (up to 45%) capable of enhancing penetration to deep tissues and to systemic circulation. It is purposed that the alcohol fluidises the ethosomal lipids and stratum corneum bilayer lipids thus allowing the soft malleable ethmosomes to penetrate (50-51). In the case of ethmosomes encapsulating drugs the higher positive potential imparted by the drug can improve skin attachment of the vesicles contribute to superior drug delivery properties (52).
Transferosomes
Transferosomes are vesicles composed of phospholipids as their main ingredients with 10-25% surfactants and 3-10% ethanol. The surfactants molecule acts as “edge activator” conferring ultradeformability of on the transferosomes, which reportedly allow them to squeeze through the channels in the stratum corneum that are less than one-tenth the diameter of transferosomes (53). The driving force for penetration into the skin is the “Transdermal gradient” caused by the difference in water content between the respectively dehydrated skin surface (approximately 20% water) and the aqueous viable epidermis (close to 100%) (53). For vesicles to remain swollen, they must follow local hydration gradient and penetrate into hydrated and deeper skin layers of viable epidermis and dermis. Traditionally liposomes are expected to confine to surface or upper layers of stratum corneum, where they dehydrate and fuse with skin lipids. Secondly transferosomes work best under in vivo conditions. Vesicles must adapt their size and/or shape, dependent on bilayer stability and elasto-mechanics, to overcome an otherwise confining pore. Ultra deformable lipid vesicles (transferosomes) can penetrate the skin and does not causes any changes in semi-permeable barriers that remain unfragmented after delivery (55).
Niosomes
These are vesicles composed of non ionic surfactants that have been evaluated as carrier for a number of drug and cosmetic application. In fact, if compared with conventional liposomes (phospholipids) niosomes (non ionic surfactant vesicles) offer higher chemical stability, lower costs, and great availability of surfactant classes .They are thought to improve the horny layer properties; both by reducing transepidermal water loss and by increasing smoothness via replenishing lost skin lipids(44).
Solid - Lipid nanoparticles
These are made up of solid lipids. Their size ranges from 50- 1000 nm (56). They can also be stabilised by surfactants or polymers. There are mainly three structures: homogenous matrix, drug enriched shell and drug enriched core. They can protect active components against chemical degradation and modulate compound release (57).
It is thought their enhanced skin penetration is primarily due to an increase in skin hydration caused by the occlusive film formed on the skin surface by the SLN. A 31% increase in skin hydration has been reported following 4 weeks application of SLN-enriched cream (58).
Aspasomes
Ascorbyl palmitate formed vesicles (Aspasomes) in presence of cholesterol and charge inducer dicetyl phosphate, encapsulating azidothymidine solution. The antioxidant potency of aspasome was much better than that of ascorbic acid. Thus, it can find applications as drug delivery system in disorders implicated with reactive oxygen species. Aspasomes enhanced the transdermal permeation of azidothymidine. The antioxidant property and skin permeation enhancing property indicate a promising future for aspasome as a carrier for transdermal drug delivery system (59).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Horney layer modified
Hydration
Water is the most widely used and safest method to increase skin penetration of both hydrophilic and lipophilic permeants The water content of the stratum corneum is around 15 to 20% of the dry weight but can vary according to humidity of the external environment. Additional water within the stratum corneum could alter permeants solubility and thereby modify partitioning from the vehicle into the membrane. In addition, increased skin hydration may swell and open the structure of the stratum corneum leading to an increase in penetration, although this has yet to be demonstrated experimentally. Hydration can be increased by occlusion with plastic films; paraffin, oils, waxes as components of ointments and water-in-oil emulsions
that prevent transepidermal water loss; and oil-in-water emulsions that donate water. Of these, occlusive films of plastic or oily vehicle have the most profound effect on hydration and penetration rate (36,38, 39,60).
Permeation enhancers:- mainly classified as chemical enhancers and biochemical enhancers. These are compounds which promote skin permeability by altering the skin as a barrier to the flux of a desired penetrant (61).
Chemical enhancers
Mechanism of chemical penetration enhancement
Penetration enhancers may act by one or more of three main mechanisms:
1. Disruption of the highly ordered structure of stratum corneum lipid.
2. Interaction with intercellular protein.
3. Improved partition of the drug, coenhancer or solvent into the stratum corneum (62).
Classification of Chemical penetration enhancers
1. Sulphoxides- DMSO, DMF.
2. Azones- 1-dodecylazacycloheptan-2-one
3. Pyrrolidones- N-methyl-2-pyrolidone
4. Essential oil, terpenes and terpenoids-sesquiterpene oil, L-menthol
5. Oxazolidinones- 4-decyloxazolidin-2-one
6. fatty acids- lauric acid, myristic acid and capric acid
7. glycol- diethylene glycol and tetraethylene glycol
8. nonic surfactant- polyoxyethylene-2-oleyl ether, polyoxy ethylene-2-stearly ether (63).
Biochemical enhancers
Recently, peptides have been examined as enhancers of skin permeability. In one approach, phage display was used to screen a library of peptides, which yielded an 11-amino acid synthetic peptide that increased transdermal delivery of insulin in diabetic rats (64). It has shown that a natural pore-forming peptide, magainin, can be used to increase skin permeability by a mechanism proposed to target bilayer disruption in stratum corneum lipids and not in deeper tissue. The magainin was only effective when used in synergistic combination with a surfactant chemical enhancer, which served the dual purpose of increasing skin permeability to the drug as well as increasing penetration of magainin into the stratum corneum .In these examples, the highly specific bioactivity enabled by peptide chemistry can enable delivery via targeted routes through the skin (65).
Skin stretching
The authors claim that a tension of about 0.01 to 10 mP results in the reversible formation of micro pathways The efficiency of the stretching process was demonstrated by monitoring the delivery of a decapeptide (1 kDa) across the skin of hairless guinea pigs using a microprotrusion array. The results of the study showed that the bi-directional stretching of skin after microprotrusion piercing, allowed the pathways to stay open (i.e. delayed closure) hence facilitating drug permeation to a greater extent (27.9 ± 3.3 μg/cm2 h) than in the control group (9.8 ± 0.8 μg/cm2 h), where the skin was not placed under tension after Microneedles treatment (66,67).
Microneedles array
Microfabricated microneedles are devices which are hybrids of the hypodermic needle and transdermal patch through the use of microscopic needles that can deliver the drug effectively (like a hypodermic needle). Their small size offers the potential advantages of delivering large molecules across the stratum corneum without extreme pain to the patients (68). The microneedles concept employs an array of micron-scale needles that can deliver drug into the epidermis and dermis, which ultimately leads to uptake by the capillaries for systemic delivery but not so far that microneedles hit the nerves. This is the reason for the device being less painful to patients. The most common material used for microfabrication of needles is silicon. A broad range of compounds such as calcein (623 Da), insulin (6000Da), BSA (66000Da) and polymeric nanoparticles are delivered at significant rates through skin permeabilized by micro fabricated microneedles (69, 70). Approach of drug targeting involves
• pretreatment of skin with microneedles, followed by application of a transdermal patch for extended drug delivery through the permeabilized skin (71).
• Another approach involves coating or encapsulating drug onto or within microneedles. Upon dissolution of the coating or the needle itself, the drug cargo is released within the skin as a bolus or possible controlled-release delivery (72, 73-75).
Microfabrication tools have been leveraged to make microneedles using methods suitable for low-cost, high-volume manufacturing, which is critical to impacting medicine as a disposable device.
Follicular delivery
Recent studies have re-examined the long held assumption that the follicles occupy approximately 0.1% of the surface area of human skin. Otberg et al. showed that follicular number, opening diameter and follicular volume are important considerations in drug delivery through these appendages and indeed the forehead provides 13.7mm2/cm2 as the follicular infundibula, i.e. approximately 13.7% of the surface area of the forehead is available as follicles and a number of hydrophilic drugs can be delivered by this route (3).
RF Microchannels
RF ablation is a well-known medical technology used to eliminate living cells. RF ablation is performed by conducting an alternating electrical current at a frequency higher than 100 KHz (radio frequency) through a particular tissue. The alternating current induces ionic vibrations in the vicinity of the electrode, resulting in heat. This, in turn, leads to water evaporation and cell ablation. RF MicroChannels are created by placing against the skin an array of closely spaced, tiny electrodes of very precise dimension. The alternating electrical current passing through the microelectrodes ablates the cells underneath each electrode, forming microscopic passages in the epidermis and outer dermis. These RF Microchannels span only the outer layers of the skin, where there are no blood vessels or nerve endings, thus minimising skin trauma and unpleasant sensations. The whole process is completed within seconds. Immediately after formation, the microchannels fill with interstitial fluid, which is responsible for heir hydrophilic nature. As a result, RF Micro Channels serve as aquatic channels into the inner layers of the skin that are embedded in the hydrophobic surroundings of the SC. The microchannels may last up to 24 hours, enabling prolonged drug delivry. After 36 hours, the drug delivery rate drops back to values characteristic of intact skin (76).
Macroflux
The system incorporates a titanium microprojection array that creates superficial pathway through the skin barrier layer to allow transportation of therapeutic proteins and vaccines or access to the interstitial fluids for sampling. Macroflux has an area of up to 8cm2 and contains as many as 300 microprojection per cm2 with individual micro projection length being < 200μm. A coating process is used to apply drug to the tip of each microprojection in the array. Three types of Macroflux have been designed and tested in preclinical studies (77). They include,
• Dry-Coated Macroflux system for short duration administration that consist of a drug coated microprojection array adhered to a flexible polymeric adhesive backing.
• D-TRANS® Macroflux system for short duration administration that consist of a microprojection array coupled with a drug reservoir.
• E-TRANS® Macroflux system for pulsatile or on demand delivery that include a microprojection array coupled with an electrotransport system (78).
Microblades
The need for such a device existed because it was hypothesized that once a drug penetrated through stratum corneum with the aid of the device, permeation through the remaining layers could proceed readily (79). The apparatus basically consists of a cutter having a plurality of microprotrusions having a height chosen with respect to the layer of skin that is to be disrupted and a „stop? for preventing the apparatus from penetrating the skin beyond a predetermined distance. As advancement to the basic technique, a microblade device along with negative pressure was patented for the Percutaneous sampling of an agent. The device was designed to optionally include a drug-sensing element. The angle of leading edge was kept between 10º-40º or the convex/ concave shaped microblades were used. It was concluded that curving of microblade?s tips outside the plane of microblade provided better anchoring (80). Another device comprising of a piercing member having plurality of microblades with 25-400μm length and provision for applying partial vacuum in the range of 0.1-0.8 atm over a period of about 2-30 sec was designed for piercing the stratum corneum for body fluid withdrawal (81).
Electrically driven methods
Ultrasound
It is typically defined as sound waves whose frequency is too high for perception by the human ear. Ultrasound (sonophoresis, phonophoresis and ultraphonophoresis) is a technique for increasing the skin permeation of drugs using ultrasound (20 KHZ to 16 MHZ) as a physical force (82). In this technique, the drug is mixed with a coupling agent usually a gel but sometimes a cream or ointment is used which transfers ultrasonic energy from the device to the skin through this coupling agent (83). Ultrasound equipment can be broadly classified as either an imaging (diagnostic equipment) or therapeutic equipment. The therapeutic equipment is used for LFS purpose due to its low frequency and adjustable modes. Equipment consist of a voltage generator, transducer and generating ultrasound (84). The mechanism of transdermal skin permeation involves disruption of the stratum corneum lipids, thus allowing the drug to pass through the skin. A corresponding reduction in skin resistance was observed due to cavitation, microstreaming and heat generation (85, 86).
Iontophoresis
Iontophoresis can be defined as the process in which the flux or rate of absorption of ionic solutes in to or through skin is enhanced by applying a voltage drop / electric field across the skin. The amount of compound delivered is directly proportional to the quantity of charge passed; it depends on the applied current, the duration of current application and the area of skin surface in contact with the active electrode compartment (87, 88).
Mechanism of penetration enhancement by Iontophoresis
• Firstly, it is purposed that drugs are forced across the skin by simple electronic repulsion of similar drugs.
• Secondly, electric current enhances permeation by inhibiting the skin ability to perform its protective barrier function.
• It causes water a very effective penetration enhancer, to enter the stratum corneum by electro-osmosis. The dissolved drug can be carried across the skin along with the penetration enhancer, to enter the stratum corneum by electro-osmosis (89).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Electroporation
This method involves the application of high voltage pulses to the skin, which has been suggested to induce formation of transient pores. The mechanism of penetration is the formation of transient pores due to electric pulses that subsequently allow the passage of macromolecules from the outside of the cell to the intracellular space via a Combination of possible processes such as diffusion and local electrophoresis (90-92). In the first technique, one of the injector was donor electrode and the other injector was the return or counter electrode. The second technique comprised of injectors serving the purpose of donor electrodes. The first technique utilized one injector for applying an electric field to the surface and the other injector was in contact with the tissue and provided electric current in conjunction with one or more electrodes. In the second case, needle free injector introduced a conductive fluid as a jet through an opening in an array electrode, which contained multiple positive and negative electrodes similar to micropatch electrode. In addition, optionally, the apparatus included a means for controlling the amount of current passing from the device and through the contacted surfaces. The electric field generated pulses of at least 50 V in about 100 m sec to 100 m sec and the pulses were either monopolar /bipolar. Enhanced permeation of polynucleotide was obtained using this technique (93).
Magnetophoresis
This method involves the application of a magnetic field which acts as an external driving force to enhance the diffusion of a diamagnetic solute across the skin. Skin exposure to a magnetic field might also induce structural alterations that could contribute to an increase in permeability. In vitro studies by Murthy showed a magnetically induced enhancement in benzoic acid flux, which was observed to increase with the strength of the applied magnetic field (94).
Medicated Tatttos
Medicated Tattoo (Med-Tat) is a modification of temporary tattoo which contains an active drug substance for transdermal delivery. Med–Tats are applied to clean, dry skin in the same manner as traditional temporary tattoos and, according to Lipper – Man Ltd, are not unsightly but rather are attractive and fun to wear. There is no predetermined duration of therapy for Med–Tats; instead, the manufacturer provides a color chart that can be compared to the color of the patient?s tattoo to determine when the tattoo should be removed. This visual comparison, which relies on the dyes incorporated into the patch, introduces a significant amount of interpatient variability.
Drugs and other compounds used in Med-Tats prototypes include acetaminophen and vitamin C. The main advantage of medicated tattoos is the delivery of drugs to children who cannot tolerate more traditional dosage forms (95).
Complexes
Complexation of drugs with cyclodextrins has been used to enhance aqueous solubility and drug stability. Cyclodextrins of pharmaceutical relevance contain 6, 7 or 8 dextrose molecules (_-, _-, _-cyclodextrin) bound in a 1,4- configuration to form rings of various diameters. The ring has a hydrophilic exterior and lipophilic core in which appropriately sized organic molecules can form non-covalent inclusion complexes resulting in increased aqueous solubility and chemical stability. Derivatives of _- cyclodextrin with increased water solubility (e.g. hydroxypropyl-_- Cyclodextrins) are most commonly used in pharmaceutical formulation. As flux is proportional to the free drug concentration, where the Cyclodextrins concentration is sufficient to complex only the drug that is in excess of its solubility, an increase in flux might be expected However, at higher cyclodextrin concentrations, the excess cyclodextrin would be expected to complex free drug and hence reduce flux. Skin penetration enhancement has also been attributed to extraction of stratum corneum lipids by cyclodextrins (96, 97)
Thermophoresis
Heat is expected to enhance the transdermal delivery of various drugs by increasing skin permeability, body fluid circulation, blood permeability, rate-limiting membrane permeability, and drug solubility. According to Kligman, diffusion through the skin, as elsewhere, temperature-dependent process, so raising the skin temperature should add thermodynamic. Drug solubility, both in the patch formulation and within the skin increase with a rise in temperature. This technology is known as Controlled Heat-aided Drug Delivery (CHADD) system. CHADD system is a small heating unit that can be placed on top of a traditional patch. The disadvantage of this technology is that heat can slightly compromise the barrier function of the skin (98).
Metered-Dose Transdermal Spray (MDTS)
This MDTS can be classified, as an enhanced, passive TDD system (99). It is a topical solution made up of a volatile cum non-volatile vehicle containing the drug dissolved as a single-phase solution. A finite metered - dose application of the formulation to intact skin results in subsequent evaporation of the volatile component of the vehicle, leaving the remaining non-volatile penetration enhancer and drug to rapidly partition into the stratum corneum during the first minute after application, resulting in a stratum corneum reservoir of drug and enhancer100. The MDTS has the following potential advantages:
1. Enhanced passive tdds with little or no skin irritation primarily as a result of its nonocclusive nature.
2. Improved cosmetic acceptability
3. Dose flexibility
4. Simplicity of manufacture (99).
References
1. Tanwar T, Marks R. Delivering drugs by the transdermal route: review and comments. Skin research and technology. 2008; 14: 249-260.
2. Songkro S. An overview of skin penetration enhancers: penetration enhancing activity, skin irritation and mechanism of action. 2009; 31(3): 299-321.
3. Soni M, Kumar S, Gupta G.D. Transdermal drug delivery: a novel approach to skin permeation. Jour of pharmacy research. 2009; 2(8):1184-1190.
4. Miller MA, Pisani E. The cost of unsafe injections. Bull World Health Organ. 1999;77: 808–811.
5. Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304: 247–268.
6. Guy RH, Hadgraft J, editors. New York: Marcel Dekker; 2003. Transdermal Drug Delivery.
7. Williams A. London: Pharmaceutical Press; 2003. Transdermal and Topical Drug Delivery.
8. Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124.
9. Bronaugh RL, Maibach HI, editors. Edn. 4th. New York: Marcel Dekker; 2005. Percutaneous Absorption.
10. Foldvari M, Babiuk S, Badea I. DNA delivery for vaccination and therapeutics through the skin. Curr Drug Deliv. 2006;3:17–28.
11. Sage H.B. In ;Swarbrick J. and Boylan J. C., Eds., Encyclopedia of pharmaceutical technology , vol 8, Mercel Dekker, New York, 217.
12. Rathbone M.J, Hadgraft J, Roberts M.S. Modified release drug delivery technology. Mercel Dekker;2003. Dermal and tyransdermal delivery.
13. Mollagard, B. And Hoelgaard, A. (1983). PERMEATION OF Estradiol through the skin effect of vehicles. Acta Pharm. Suec, 20:443-450.
14. Barry B.W. (1987).mode of action of penetration enhancers in human skin J. Control release, 6:85-87.
15. Gaur PK, Mishra S, Dave PK. Transdermal drug delivery system: a review. Asian J Pharm Clin Res. 2009; 2(1): 14-20.
16. Dhamecha DL, Rathi AA, Saifee M, Lahoti SR, Dehghan HG. Drug vehicle based approach of penetration enhancement. Int J Pharmacy Pharm Sci. 2009; 1(1):24-46
17. Tiwari A.K, Sapra B, Jain S. Innovation in transdermal drug delivery: formulation and techniques. Recent patent on drug discovery and formulation. 2007;1(1):23-36.
18. Kumar R, Singh J, Philip A, Pathak K. Patented active transport transdermal drug delivery technologies. The pharma review. 2007: 133-138.
19. Chein YW and Chi shun lee. Transdermal Drug Delivery System with Enhanced skin Permeability. In: Ping IL and William RG, editors.Controlled Released Technology-Pharmaceutical Application.Washington DC: American Chemical society; 1987. p. 81-299.
20. Mishra AN. Transdermal drug delivery. In: Jain NK, editors. Controlled and Novel Drug Delivery. New Delhi: Varghese Publication; 1998. p. 100-129.
21. Chein YW. Advance in Transdermal Systemic Medication. In: Chien YW, editors. Transdermal Controlled Systemic Medication. New York: Marcel Dekker Inc.; 1987.p.1-24.
22. Brain KR, Walters KA, Watkinson AC.Methods for studying percutaneous absorption. In: Walters KA, editors. Dermatological and transdermal formulations. New York: Marcel Dekker Inc.; 2002. p. 241-247.
23. Ranade V. Dug delivery systems. Transdermal drug delivery. Clinical Pharmacol 1991; 31(6): 401–418.
24. Ranade VV, Hollinger MA, Drug Delivery Systems.2nd ed: CRC press publication; 2000.p. 207-248.
25. Sloan KB, Wasdo S. Med. Res. Rev., 2003;23:763-93
26. Beall H, Prankerd R, Sloan K. Transdermal delivery of 5- fluorouracil (5-FU) through hairless mouse skin by 1-alkyloxycarbonyl- 5-FU prodrugs: Physicochemical characterization of prodrugs and with transdermal delivery. Int J Pharm 1994;111:223–233.
27. Stinchcomb, A.L.,Swaan,P.W.:US20036569449B1 (2003).
28. Kiptoo PK, Hamad MO, Crooks PA, Stinchcomb AL. Enhancement of transdermal delivery of 6-beta-naltrexol via a codrug linked to hydroxybupropion. J Control Release. 2006;113:137–145.
29. Higuchi T. Physical chemical analysis of percutaneous absorption process. J Soc Cos Chem 1960,11:85-97.
30. Daniels R. Strategies for skin penetration enhancement. Skin Care Forum [online]. 2004 [cited 2004 Aug].
31. Kemken S, Ziegler A, Muller BW.Influence of supersaturation on thermodynamic effect of bupronolol after dermal administration using microemulsions as vehicle.Pharmaceutical Research 1992; 9:554-558.
32. Pellett MA, Hadgraft J, Roberts MS. The back diffusion of glucose across human skin in vitro. Int J Pharm 1999;193:27–35. Pellett MA, Davis AF, Hadgraft J. Effect of supersaturation on membrane transport: 2. Piroxicam.Int J Pharm 1994; 111:1-6.
33. Pellett MA, Hadgraft J, Roberts MS. Supersaturated solutions evaluated with an in vitro stratum corneum tape stripping technique. Int J Pharm 1997; 151: 91-98.
34. Dias MMR, Raghavan SL, Pellett MA, Hadgraft J. The effect of cyclodextrins on the permeation of diclofenac from supersaturated solutions. Int J Pharm 2003; 263: 173–181.
35. Moser K, Kriwet K, Kalia YN, Guy RH. Enhanced skin permeation of a lipophilic drug using supersaturated formulations. J Control Release 2001; 83: 245–253.
36. Barry BW. Is transdermal drug delivery research Still important today? DDT (2001) 19(6): 967-971.
37. Origins of Life and Evolution of the Biosphere, Volume 40, Numbers 4-5, October 2010 , pp. 347-497(151)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
38. Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001; 14: 101-114.
39. Benson HAE. Transdermal Drug Delivery: Penetration Enhancement Techniques. Current Drug Delivery (2005) 2: 23-33.
40. Stott P W, Williams AC, Barry BW. Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen. J Control Release 1998; 50: 297–308.
41. Woolfson AD, Malcolm RK, Campbell K, Jones DS, Russell JA. Rheological, mechanical and membrane penetration properties of novel dual drug systems for percutaneous delivery. J Control Release 2000; 67: 395–408.
42. Stott PW, Williams AC, Barry BW. Mechanistic study into the enhanced transdermal permeation of a model -blocker, propranolol, by fatty acids: a melting point depression effect. Int J Pharm 2001; 219: 161– 176.
43. Kang L, Jun HW, McCall JW. Physicochemical studies of lidocaine–menthol binary systems for enhanced membrane transport. Int J Pharm 2000; 206: 35–42.
44. Vyas SP, Khar RK. Controlled drug delivery system.1st ed: Vallabh prakashan. 2005. p. 417-426.
45. Yu, et al topical delivery of marine gene, Invest, Dermatol,1999;88;569-573.
46. Kost J, Katz N, Shapiro D, Herramann T, Kellog S, Warner N, Custer L. Ultrasound skin permeation pretreatment to accelerate the onset of topical anaesthesia. Proc Inter Symp Bioact Mater 2003.
47. Burkoth TL, Bellhouse BJ, Hewson G, Longridge DJ, Munddle AG, Sarphie DF. Transdermal and transmucosal powder drug delivery. Critical Reviews Therapeutic Drug Carrier System 1999; 16:331-334.
48. The two phase of needle- free injection technology market” Drug delivery technology. Volume 4 no. 6, 2004, 38-43.
49. Kumar R, Singh J, Philip A, Pathak K. Patented active transport transdermal drug delivery technologies. The pharma review. 2007:133-140.
50. Touitou E, Godin B. Skinnonpenetrating sunscreens for cosmetic and pharmaceutical formulations. Clinics in Dermatology 2008; 26:375–379.
51. Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, Levi-Scha F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials 2001; 22:3053–3059.
52. Touitou, E., Dayan, N., Bergeison, L., Levi- Schaffer F. And Piliponsky A. Novel lipid vesicular system for enhanced delivery. J Liposomes Res. 1998, 8, 112-14.
53. Ceve, G, Crit. Rev. Ther. Drug Carrier Syst., 1996, 13, 257-388.
54. Benedicte AI, Bergh VD, Wertz PW, Junginger HE, Bouwstra JA. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements, Int J Pharm 2001; 217:13–24.
55. Cevc G, Schatzlein A, Richardsen H. Ultradeformable lipid vesicles can penetrate the skin and other semipermeable barriers unfragmented. Evidence from double label CLSM experiments and direct size measurements. Biochemical et Biophysical Acta. 2002; 1564:21– 30.
56. Muller RH, Radtke M, Wissing SA. Solid lipid nanopaerticles and nanostructured lipid carriers in cosmetic and dermatologic preparations. Adv Drug Deliv Rev 2002; 54 (1): 77-98.
57. Tadros TF, Future Developments in cosmetic preparations. Int J Cos Sci 1992; 14 (3):93-111.
58. Lee PW, Peng SF, Su CJ, Mi FL, Chen HL, Wei MC, Lin HJ, Sung HW. The use of biodegradable polymeric nanoparticles in combination with a low-pressure gene gun for transdermal DNA delivery. Biomaterials 2008; 29:742–751.
59. Gopinath D, Ravia D, Raoa BR, Apte SS, Renuka D, Rambhaua D. Ascorbyl palmitate vesicles (Aspasomes): formation, characterization and applications. Int J Pharm 2004; 271:95–113.
60. Modesti M, Dall Acqua C, Lorenzetti A and Florian E. Mathematical model and experimental validation of water cluster influence upon vapour permeation through hydrophilic dense membrane. J Membrane Science (2004) 229: 211–223.
61. Chopda G. Transdermal drug delivery system: A review. 2006. 4(1). Downloaded from pharmainfo.net.
62. Barry BW, William AC. In: Swarbrick J (ed), Boylon JC. Encyclopedia of pharmaceutical technology Vol II, Marcel Dekker: Inc new York 1995, pp 49-93
63. Pathan IB, Setty CM. Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pharm Res. 2009; 8(2):173-179
64. Chen Y, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006;24:455–460.
65. Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by magainin pore-forming peptide. J Control Release. 2007;122:375–383
66. Cormier, M., Trautman, J., Kim, H.L., Samiee, A.P., Ermans, A.P., Edwards, B.P., Lim, W.L. and Poutiatine, A. (2001) “Skin treatment apparatus for sustained transdermal drug delivery”, Patent (Serial Number WO 01/41864 A1).
67. Neukermans, A.P., Poutiatine, A.I., Sendelbeck, S., Trautman, J., Wai, L.L., Edwards,
68. B.P., Eng, K.P., Gyory, J.R., Hyunok, K.L., Lin, W.Q. and Cormier M (2001) “Device and method for enhancing microprotrusion skin piercing”, Patent (Serial Number WO 0141863) Jones S A. Evolution of transdermal drug delivery: Recent progress in active drug delivery technologies has helped miniature, powerful device to generate required clinical responses.www.pharmaquality.com.
69. ALZA website, https://www.alza.com/, Retrieved.
70. McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proceedings of the National Academy of Sciences of the United States of America 2003;100: 13755-60.
71. McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc Natl Acad Sci USA, 2003 Nov 25, Epub (2003).
72. Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res 19, 2002, 63–70.
73. Miyano T, Tobinaga Y, Kanno T, Matsuzaki Y, Takeda H, Wakui M, et al. Sugar microneedles as transdermic drug delivery system. Biomedical Microdevices 7, 2005, 185–188.
74. Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res 23, 2006, 1008–1019.
75. Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Rel 117, 2007, 227–237.
76. Levin G., R.F Microchannel based transdermal delivery. Innovations in pharmaceutical technology: 66-68.
77. Lin W, Cormier M, Samiee A. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux®) technology. Pharm Res. 2001; 18: 1789- 93.
78. Rathbone M J, Hadgraft J, Roberts M S (eds.) Modified release drug delivery technology. New York, Marcel Dekker, Inc., 2004; vol.126: 471-619.
79. Gerstel, M.S., Place, V.A.: US19763964482 (1976).
80. Cormier, M.J. N., Theeuwes, F.: US20036537264B1 (2003).
81. Lin, W.Q., Cormier, M. J. N., Theeuwes, F.: US20036562014B2 (2003).
82. Mitragotri S, Blankschtcin D, Langer R. Ultrasound mediated transdermal protein delivery. Science 1995;269:850-853.
83. Mormito Y, Mutoh M, Ueda H, Fang L, Hirayama K, Atobe M, Kobayashi D. Elucidation of the transport pathway in hairless rat skin enhanced by low frequency sonophoresis based on the solute water transport relationship and confocal microscopy. J. Control Release 2005; 103: 587-597.
84. Anand S, Nanda S. Sonophoresis: Biophysical basis of transdermal drug delivery. In: Jain N.K, editor. Controlled and novel drug delivery. 1st ed. New Delhi: CBS Publishers and distributers;2002:211-213.
85. Mitragotri S, Kost J. Transdermal delivery of heparin and low-molecular weight heparin using low-frequency ultrasound. Pharmaceutical Research 2001; 18: 1151-56.
86. Tang H, Wang C C J, Blankschtein D, Langer R. An investigation of the role of cavitation in low frequency ultrasound mediated transdermal drug transport. Pharmaceutical Research 2002; 19: 1160-69.
87. Srinivasan, V., Higuchu , W.I., Su, M.H., J. Controlled Release.1989;10:157.
88. Thuyduong T. Le. “ Iontophoresis Drug Delivery Electrode” Biomedical Engineering – University of Rhode island. October 2:2000.
89. Roberts, M., S., Lai, P.,M., Cross, S.E., Yoshida , N., H., Mechanism of transdermal druf delivery, Mercel Dekker, Newyork, 1997, 291-349.
90. Prausnitz M R. A practical assessment of transdermal drug delivery by skin electrporation, Adv. Drug Deliv Rev. 1999; 35: 61-76.
91. Benga A K, Bose S and Ghosh T, K, Iontophoresis and electroporation: comparisions and contrasts. Int J. Pharm., 179: 1-19.
92. Zhang L, Li L, Hoffmann GA, Hoffman RM. Depth targeted efficient gene delivery and expression in the skin by pulsed electric fields: an approach to gene therapy of skin aging and other diseases. Biochem. and Biophy. Res. Comm. 1996; 220: 633-36.
93. Hofmann, G.A., Rabussay, D.P., Zhang, L.: US20036520950B1 (2003).
94. Murthy, S.N. (1999) “Magnetophoresis: an approach to enhance transdermal drug diffusion”, Pharmazie. 54, 377-379.
95. Lipper-Man Ltd., Competitive advantages
96. Challa R, Ahuja A, Ali J, Khar R. Cyclodextrins in Drug Delivery: An Updated Review. AAPS PharmSciTech 2005; 06(02): E329- E357.
97. Kear CL, Yang J, Godwin DA, Felton LA. Investigation into the Mechanism by Which Cyclodextrins Influence Transdermal Drug Delivery. Drug Dev Ind Pharm 2008; 34(7): 692 – 697.
98. Kligman AM: A biological brief on percutaneous absorption. Drug Dev Industr Pharm 9:521-560, 1983.
99. Rathbone M J, Hadgraft J, Roberts M S (eds.) Modified release drug delivery technology. New York, Marcel Dekker, Inc., 2004; vol.126: 471-619.
100. Morgan T M, Sullian H, Reed B L, Finnin B C. Transdermal delivery of estradiol in postmenopausal women with a novel topical aerosol. J. Pharm Sci.1998; 87:1226-28.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









