About Authors: Bindi G. Chavda1 ,Vipul P. Patel2, Tushar R. Desai3,
1. Authour, R.K. College of Pharmacy, Kasturbadham, Rajkot.
2. Assistant professor (M.Pharm), R.K. College of Pharmacy, Kasturbadham, Rajkot.
3. Principal (Ph.D), Department of pharmacology, R.K. College of Pharmacy, Kasturbadham, Rajkot.
Reference ID: PHARMATUTOR-ART-1047
ABSTRACT
Chewing gums are mobile drug delivery systems. It is a potentially useful means of administering drugs either locally or systemically via, the oral cavity. The medicated chewing gum has through the years gained increasing acceptance as a drug delivery system. Several ingredients are now incorporated in medicated chewing gum, e.g. Fluoride for prophylaxis of dental caries, chlorhexidine as local disinfectant, nicotine for smoking cessation, aspirin as an analgesic, and caffeine as a stay alert preparation. In addition, a large number of chewing gum intended for prevention of caries, xerostomia alleviation, and vitamin/ mineral supplementation are currently available. Medicated chewing gums are solid, single dose preparations with a base consisting mainly of gums that are intended to be chewed but not swallowed. Today improved technology and extended know how have made it possible to develop and manufacture medicated chewing gum with predefined properties. Consequently today chewing gum is a convenient drug delivery system, which is appropriate for a wide range of active substances.
[adsense:336x280:8701650588]
INTRODUCTION
Chewing gum is being used worldwide since ancient times after man experienced the pleasure of chewing a variety of substance. One thousand years ago the Mayan Indians chewed tree resin from the sapodilla tree in order to clean their teeth and freshen their breath. Shortage of natural gum bases during World War II enhanced development of the synthetic gum bases that are used today. Chewing gum can be used as a convenient modified release drug delivery system. Medicated chewing gums are currently available for pain relief, smoking cessation, travel illness, and freshening of breath. In addition, a large number of chewing gum intended for prevention of caries, xerostomia alleviation and vitamin / mineral supplementation are currently available. The first commercial chewing gum “State of Maine pure spruce gum” was marketed in 1948 in the U.S.A. The first patent was filed in 1869. The gum was intended as dentifrices but it has never been marketed. The first Medicated chewing gum “Aspergum” was launched in 1928. This chewing gum is still available and contains acetylsalicylic acid. Another commercially available medicated chewing gum is dimenhydrinate – containing chewing gum for motion sickness. However, chewing gum did not gain acceptance as a reliable drug delivery system until 1978, when nicotine chewing gum became available. Today improved technology and extended know how have made it possible to develop and manufacture medicated-chewing gum with pre-defined properties. Consequently, today chewing gum is a convenient drug delivery system, which is appropriate for a wide range of active substances. Medicated chewing gum offers advantages in comparison to conventional oral mucosal and oral dosage forms both for (a) local treatment (b) systemic effect after absorption through the buccal and sublingual mucosal and from the gastrointestinal tract. Chewing gum can be retained in the oral cavity for a long period and, if the drug is readily absorbed across oral mucosa, chewing gum can provide a fast onset time for a systemic effect and the potential for avoidance of gastrointestinal and hepatic first – pass metabolism of susceptible drugs. Generally, medicated chewing gum has a good stability, the medicine can be taken easily and directly without the prerequisite of water, and if required, prompt discontinuation of medication is possible. Physiochemical properties of the drug like aqueous stability, pKa value, distribution between gum/ saliva, product properties like, composition, mass, manufacturing process and the process of chewing i.e. chewing time, chewing rate, affects the release of drugs from the medicated chewing gum. Varying the formulation and manufacturing process, chewing gum as a drug delivery system can be formulated for an extended period of time.
HISTORY
People worldwide have chewed on natural materials for hundreds of years. Some of the first substances used as chewing gums by early people were frankincense, mastic and the chili of the manilkara zapodilla tree. Today combinations of materials with other agents are still being used in the production of chewing gum. Frankincense is probably the most well-known resin tree, obtained from the Boswellia tree and it is frequently mentioned in the Bible. The ancient Egyptians in their religious rites also used it, while nomads in Somalia used to wear it in ponches with the hope that this resin could quench their thirst and compensate for the scarcity of water in their dry land.
Mastic is a resin taken from a tree. The word “mastic” is probably derived from the Greek “ mastichon”, which means, “ to chew” and it is also the root of the English word “ masticate”. This substance is formed from the resin contained in the bark of the mastic tree found mainly in Greece and Turkey. Greek women favored chewing mastic gum to clean their teeth and sweeten their breath. Mastic is still widely chewed today by many Greeks and in Middle East. Actually, some of the properties and uses of rubber were discovered by the Native South American long before the voyages of Columbus in 1492 and then made the knowledge available to Europe. The Indians of New England taught American colonists to quench their thirst by teaching them how to chew the gum-like resin that forms on spruce trees when its bark is cut. In the early 1800s, lumps of this spruce gum were sold in the eastern United States, making it America’s first commercial chewing gum. Sweetened paraffin wax became an acceptable alternative around 1850. Modern chewing gum evolved from chicle – based gum brought to the United States in the early 1860s. Chicle is derived from the milky juice (latex) of the sapodilla tree that grows in tropical rain forests of Central America. Paraffin, originally discovered in 1830, was an option for chewing gum base. But the search for a better material continued.
An Ohio dentist used rubber to create a gum product for jaw exercise and gum stimulation. William F. Sample was honored for his work with the first patent to manufacture chewing gum in 1869. Today, gum base is made of man-made latex and divided into two major categories: chewing and bubble gum, with the latter having more elasticity. In recent years, nonstick gum bases for chewing and bubble gums have been formulated to satisfy the needs of more consumers.
AIM AND OBJECTIVES
The aim of this review article is (1) to discuss the advantages and limitation of chewing as drug delivery system, (2) to describe the methods of preparation, evaluation, stability, release of drugs, factors affecting release, safety aspects and development with respect to the medicated chewing gums.
CHEWING GUM DOSAGE FORM FOR BUCCAL DELIVERY
Dosage forms such as mouthwashes, erodible/ chewable buccal tablets, and chewing gums allow release of drugs for only a short period and thus the reproducibility of drugs absorption is comparatively poor. Application of bioadhesive semisolid gels creates considerable technical problems in the buccal absorption. Although medicated chewing gums pose difficulties in regulating the dose administered, they still have some advantages as drug delivery devices, particularly in the treatment of diseases in the oral cavity and in nicotine replacement therapy. Some commercially available chewing gums are Caffeine chewing gum, (Stay Alert®,) and Nicotine chewing gums (e.g. Nicorette ® and Nicotinell®). The permeability of nicotine across the buccal mucosa is faster than across the skin. However, chewing gum slowly generates a steady plasma level of nicotine rather than a sharp peak as experienced when smoking. Possible swallowing of considerable amount of nicotine during chewing may lead to decreased effectiveness of the chewing gum due to first pass metabolism and gastrointestinal discomfort. It is a major challenge to optimize the dose-response relationship of nicotine administered in a chewing gum.
ADVANTAGES 12
1. Convenient – promoting higher compliance
2. Discreet- less stigmatization
3. Administration without water can be taken anywhere
4. Excellent for acute medication
5. Advantageous for patients with difficulty in swallowing tablets
6. Pleasant taste
7. Dose not requires water to swallow. Hence can be take anywhere.
8. Counteracts dry mouth: Through stimulation of the salivary secretion thereby preventing
9. Candidacies and caries
10. Highly acceptable by children
11. Excellent for acute medication
12. The active compounds absorbed at oral level avoid the hepatic circulation and the associated metabolism.
13. Gum does not reach the stomach. Hence G.I.T. suffers less from the effects of excipients.
14. The product is rapidly released from the gum after a short period mastication; some absorption takes place by directly through the oral mucosa depending on the active ingredient. Importantly not being swallowed the gum does not reach the stomach. Moreover, the stomach does not suffer from direct contact with high concentrations of active principle, thus reducing the risk of intolerance of the gastric mucosa.
15. The fraction of product reaching the stomach is conveyed by the saliva and delivered Continuously and regularly. Active substances are released from medical chewing gum during chewing and are dissolved in saliva. The release rate can be carefully controlled through the formulation of the chewing gum allowing extendedexposure in the oral cavity. Active substances that are absorbed through the buccal mucosa pass via the jugular veins directly into the systemic circulation. Due to the rich vascular supply of the buccal mucosa, measurable concentrations of active substances may be in the blood after only a few minutes of chewing and fast onset of action is thus likely to be attained. Furthermore, bioavailability may be increased, as hepatic first pass metabolism and gastrointestinal tract degradation are tract degradation are avoided for buccal-absorbed substances.
16. Aspirin, Dimenhydrinate and Caffeine shows faster absorption through MCG than tablets.
17. Consequently, a lower dosage of substance may be therapeutically sufficient, possibly resulting in a fewer side effects, and promote fast absorption. Active substances released from chewing gum are dissolved in saliva when swallowed and are, therefore, readily accessible for absorption in the gastrointestinal tract.
MANUFACTURING
1. Gum Base-Gum base is an inert and insoluble nonnutritive product used as a support for the edible and soluble of the chewing gum (sugar, glucose, poly oils and flavors) Other raw materials are generally grouped in the following classes:
2. Elastomers: including natural and synthetic rubbers. The gum base composition may contain conventional elastomer solvents to aid in softening the elastomer base component. Such elastomer solvents may comprise terpinene resins such as polymers of alpha-pinene or beta-pinene, methyl, glycerol or pentaerythritol esters of resins or modified resins and gums, such as hydrogenated, dimerized or polymerized resins or mixtures. The elastomer solvents may be employed in amounts from 5.0% to 75.0%, by weight of the gum base, and preferably from 45.0% to 70.0%, by weight of the gum base. Synthetic elastomers such as butadiene, styrene copolymers, polyisobutylene, isobutylene isoprene copolymers, polyethylene mixtures, and non-toxic vinyl polymer, such as polyvinyl alcohol are widely used bases. The molecular weight of the vinyl polymer may range from 3,000 to 94,000. The amount of gum base employed varies greatly depending upon various factors such as the type of base used, the consistency of the gum desired and the other components used in the composition to make the final chewing gum product. In general, the gum base will bepresent in amount from 5% to 94%, by weight of the final chewing gum composition. Preferably, the gum base is used in amounts from 15% to 45% and more preferably in amounts from 15% to 35% by weight of the final chewing gum composition.
3. Plasticizers: waxes, vegetable oils, glycerides. Plasticizers or softeners such as lanolin, palmitic acid, oleic acid, stearic acid, sodium stearate, potassium stearate, glyceryl triacetate, glyceryl lecithin, glyceryl monostearate, propylene glycol monostearate, acetylated monoglyceride, glycerine, natural and synthetic waxes, hydrogenated vegetable oils, polyurethane waxes, paraffin waxes, microcrystalline waxes, fatty waxes, sorbital monostearate, propylene glycol, may be incorporated into the gum base to obtain a variety of desirable textures and consistency properties.
4. Adjuvants: calcium carbonate, talc, or other charging agents are used. Mineral adjuvant such as calcium carbonate, magnesium carbonate, aluminum hydroxide, aluminum silicate, talc, tricalcium phosphate, dicalcium phosphate serve as fillers and textural agents.
5. Antioxidants: An anti- oxidant such as butylated hydroxytoluene, butylated hydroxyanisole, propyl gallate and mixtures there of, may be included as antioxidants.
6. Compression adjutants: Suitable compression adjuvant such as silicon dioxide, magnesium stearate, calcium stearate and talc can be used in medicated chewing gum for ease of compression. The alkaline earth metal phosphates and alkali metal phosphates prevent caking and balling of “High” i.e. 2 to 8% moisture- containing chewing gum compositions during grinding. Additionally, it has been discovered that maltodextrin enhances the grinding of “high” moisture-containing chewing gum compositions by absorbing moisture to allow lubrication in the gum as it separates into granules. If oil lubricants are used, it is preferred to be 0.4% to 1% by weight of the tableted chewing gum composition. The amount of glidant present in the tableted chewing gum composition is from 0.5% to 5% by weight of the tableted chewing gum composition. Those glidants useful are selected from the group consisting of alkali metal salts, talc, starch, polyhydric alcohols and mixtures. Antiadherents function to prevent tablet granulations from sticking to the faces of the punches and the die walls, but most importantly, prevent adherence of chewing gum granules from adhering to one another, a phenomenon known as blocking. Anti- adherents may be added to the chewing gum composition while the composition is in the hoppers, or subsequent to grinding and are selected from the group consisting of silicates, silicon dioxide, talc and mixtures thereof present in amount of 0.2% to 1% by weight of the tableted chewing gum composition and preferably about 0.3 to about 0.6% by weight. Generally anti-adherent is a finely divided low bulk density powder, which is preferably water insoluble. The preferred anti-adherents are fumed silica and talc. The term-fumed silica is meant to include pyrogenic silicas, micron sized silicas and hydrated silicas.
7. Sweeteners
a) Water-soluble sweetening agents: xylose, ribulose, glucose, mannose, galactose, fructose, sucrose, maltose, invert sugar partially hydrolyzed starch, dihyrochalcones, monellin, steviosides, glycyrrhizin, and sugar alcohols such as sorbitol, mannitol, hydrogenated starch hydrolsates.
b) Water-soluble artificial sweeteners: soluble saccharin salts, i.e. sodium or calcium saccharin salts,cyclamate salts.
c) Dipeptide based sweeteners: L- aspartic acid derived sweeteners such as Aspartame, Alitame, methyl esters of L-aspartyl-L phyenyl-glycerine and Laspartyl- L 2,5-dihyrophenylglycine, L-aspartyl 2,5- dihydro-L phenylalanine – L aspartyl – L (1-cyclohexen) alanine.
d) Water-soluble sweeteners: derived from naturally occurring water-soluble sweeteners, chlorinated derivatives of ordinary sugar (sucrose, known as Sucralose)
e) Protein based sweeteners: such as thaumaoccous danielli (Thaumatin I and II) In general an effective amount of sweetener is utilized to provide the level of sweetness desired, and this amount will vary with the sweetener selected and are present in amounts from 0.0025% to 90% by weight of the gum composition.
8. Coloring Agents: The coloring agents include pigments, which may be incorporated in amounts up to about 6% by weight of the gum composition, titanium dioxide may be incorporated in amounts up to about 2%. The colorants may also include natural food colors and dyes suitable for food drug and cosmetic applications.
9. Flavoring Agents: Flavoring agents suitable for use are essential oils and synthetic flavors such as citrus oils, fruit essences, peppermint oil, spearmint oil, clove oil wintergreen oil, and anise oil.
PROCESS FOR PREPARATION
Different authors have reported various processes of preparation of medicated chewing gum, that are mentioned as under:
1. Formation of a inclusion complex, which is dried and mixed granulated gum base without adding water or other solvents. The process is carried out at controlled temperature and humidity and the blended components are cold pressed to produce a final gum product. Attempts have been made to incorporate pharmaceutically active agents into chewing gum as means of administering the active agent to the subject. Traditionally, these efforts have employed common chewing gum production techniques wherein a gum base is heated until it becomes viscous or fluid mass. Additional components (such as flavors or active ingredients) then are blended into gum base. Finally, the mixture is cooled, pressed and cut to produce the final product.
2. Alternatively, various components are blended in gum slurry that is coagulated before pressing into the final product form.
3. To avoid the degradation of the active agents, coldproduced chewing gum by direct compression of the ingredients has been tried so as to retard or control the release rate of active agents along with the microencapsulated active agents.
4. Use of directly compressible chewing gum excipients: if a directly compressible chewing gum excipients is available. The limitations of melting & freezing can be overcome by the use of these. PHARMAGUM®,is one such compactable gum system developed by SPI Pharma. Pharmagum is a mixture of polyol(s) & or sugars with a chewing gum base. It is available as directly compressible powder, free flowing powder which can be compacted into a gum tablet using conventional tablet press thus enabling rapid and low cost development of a gum delivery system. It is manufactured under CGMP conditions and complies with Food Chemicals Codex specifications as well as with FDA, so they can be considered as "Generally regarded as safe" (GRAS). Pharmagum® is available in three forms namely S, M and C. Pharmagum® M has 50% greater gum base compared to Pharmagum®S. Pharmagum®S consists primarily of gumbase and sorbitol. Pharmagum®M contains gumbase, mannitol & Isomalt. Release of nicotine from directly compressible nicotine gum formulations and from Nicorette® prepared by conventional methods have shown that use of Pharmagum in formulation showed a faster release rate. Formulations made with Pharmagum® M & S, are similar to tablet in appearance. Gums formed using compressible formulation are 10 times harder and crumble when pressure is applied resulting in faster release than conventional methods.
5. Conventional/ traditional Method (Melting): Components of gumbase are softened or melted and placed in a kettle mixer to which sweetners, syrups, active ingredients and other excipients are added at a definte time. The gum is then sent through a series of rollers that form into a thin, wide ribbon. During this process, a light coating of finely powdered sugar or sugar substitutes is added to keep the gum away from sticking and to enhance the flavour. In a carefully controlled room, the gum is cooled for upto 48 hours. This allows the gum to set properly. Finally the gum is cut to the desired size and cooled at a carefully controlled temperature and humidity.
Limitations: (i) Elevated temperature used in melting restricts the use of this method for thermo labile drugs.(ii) Melting and mixing of highly viscous gum mass makes controlling of accuracy and uniformity of drug dose difficult.
PROBLEMS ASSOCIATED WITH ABOVE METHODS
1. Many pharmaceutically active agents possess unpleasant taste or odor that results in the undesirable chewing gum products. Many active agents also tend to irritate the mucosa and few others degrade rapidly, making impractical to include them in chewing gum.
2. Another problem associated with the above methods is that the gum base is heated to a fluid mass to facilitate mixing of other ingredients. Such elevated temperatures can cause degradation of heatsensitive compounds, including active agents and flavors.
3. Further, medicated gum preparations often utilize organic solvents to dissolve the active agents, it is difficult to eliminate these organic solvents from the final product and may present certain health risks if even trace amounts remain in the final dosage forms.
4. Additionally use of organic solvents in connection with industrial processes is becoming increasingly unpopular due to health and environmental considerations (e.g. risks attendant to exposure of personnel and problems in effecting proper disposal of waste solvents)
5. Risk of over dosage with MCG compared with chewable tablets or lozenges that can be consumed in a considerable number and within much shorter period of time.
6. Water can also be utilized in gum preparations but it is difficult to eliminate, especially at the relatively low temperatures that are desirable for production of chewing gum. Heating the gum mass to eliminate water is not advisable, because the gum will then become stickier which makes handling difficult and interferes with large scale, semi-or totally automated production. Conventional chewing gum compositions are difficult to form into chewing gum tablets because of their moisture content. Traditional chewing gum compositions contain 2% to 8% by weight of water.
7. Generally the chewing gum will jam the grinding machine, sticking to blades, screens and other surfaces if moisture level is not controlled. Traditional moisture levels can cause caking and balling of the gum during granulation process thereby preventing the formation of gum granules that are necessary for tableting. Alternatively, if moisture content is greater than 2% by weight, various other problems can occur, e.g. adherence to the punch press, compaction in the punch press hopper, poor flow in the feeder section and difficulties with compressibility. Thus the problems of realizing a medicated chewing gum produced by direct compression with immediate or rapid release of the active agent and efficient masking of unpleasant organoleptic characteristics of active agents remain unsolved. In view of the deficiencies of the prior methods of producing medicated chewing gum containing active agents a process has been reported that overcomes these various shortcomings and provide a novel process for producing medicated chewing gum containing inclusion complexes of cyclodextrins and active agents. This process avoids the use of organic solvents and water and is especially adapted for use with heat sensitive active agents. Further, it avoids the heated gum base and slurries too. The novel method that has been patented includes cooling the gum base grinding it into granules, which are then dry-mixed with optional excipients in the absence of water and solvents at controlled temperature and humidity, cold- pressing the mixed gum and components into the final product. The hydro-soluble and lipid soluble active agent are encapsulated in cyclodextrins and are liberated in the mouth by saliva amylases. By inclusion in cyclodextrins, the active agents are stabilized and are made more soluble. The well-developed technology used for generating the inclusion complexes is simpler and advantageous when compared to that of micro encapsulation. Furthermore, through the use of the inclusion complexes, the bioavailability of the cyclodextrinencapsulated active agents is accentuated. In addition this technique provides an excellent means of masking the unpleasant organoleptic characteristics of many active agents. One or more well-known chewing gum excipients can be added to the gum base before or after combining it with the inclusion complex. The powder containing the inclusion complexes of cyclodextrin and active agent is mixed with the processed gum base in the absence of water or organic solvents. The ratio between the inclusion complex and the processed gum based can vary widely depending on the amount of active agent that should be delivered. The medicament is added to the gum granules along with the compression aid, pharmaceutical drugs or other active agents are added in a number of forms including encapsulated, but they are preferably added in a dry state. Active agents may themselves be granulated and added in this form to the tableted chewing gum composition. Liquid water-soluble drugs can be added to a solution of modified malt dextrin and spray dried. Liquid, oil soluble drugs and active agents can be blended with the compression aid components prior to mixing with the gum granules, the liquid drug or active agent should not exceed more than 30% by weight. The advantage of the tableted gum composition as a means for dispensing drugs medicaments and other active agents is that the component is trapped between granules and not within the gum composition. Hence, it is readily bioavailable and nearly completely released upon chewing of the gum.
8. Sorbitol present in MCG formulation may cause flatulence, diarrhea.
9. Chlorhexidine oromucosal application is limited to short term use because of its unpleasant taste and staining properties to teeth and tongue.
10. Chewing gum have been shown to adhere to different degrees to enamel dentures and fillers.
11. Prolong chewing on gum may result in pain in facial muscles and earache in children.
12. Additives in gum like flavouring agent, Cinnamon can cause Ulcers in oral cavity and Licorice causes Hypertension.
STABILITY
The stability of chewing gum is comparable to that of most other solid delivery systems. Chewing gum normally contains little water (2.5%). If the water content is very critical for the stability of drug, the chewing gum can be manufactured without water (less 0.2%). This will however, often make the product hygroscopic and will affect the texture. The low water content also inhibits microbial growth in the chewing gum during storage. Furthermore, the product can be protected against oxidation by a sealed coat and by an appropriate packing. For every temperature-labile component, e.g. enzymes, the process temperature of 50-600 C during mixing may create a stability problem. It is however possible to operate the process at a lower temperature to avoid this issue.
QUALITY CONTROL
As per specifications given in European Pharmacopoeia.
1) Test for Uniformity of Content: Unless otherwise prescribed or justified and authorized medicated chewing gum with content of 2 mg or less than 2 percent of the total mass of gum comply with test.
2) Uniformity of mass: Uncoated medicated chewing gum and unless otherwise justified and authorized coated medicated chewing gum comply with the test for uniformity of mass of single- dose preparations.
3) Drug release from medicated chewing gum: It has been reported commercially that the drug release from medicated chewing gum as per the specification given in European Pharmacopoeia and is determined by applying a mechanical kneading procedure to a piece of gum placed in a small chewing chamber containing a known volume of buffer solution.
FACTORS AFFECTING RELEASE
The release rate of an active substance is determined not only by the formulation of the chewing gum but also by the properties of the active substance and of the individual chewing the gum. The chewing gum – The water content of gum base is very low and the gum binds lipophilic substances very firmly. In order to obtain the optional formulation it is possible to
1. Decrease the release rate of highly hydrophilic substances
2. Increase the release rate of lipophilic substances
3. Achieve a more complete release of lipophilic substances
4. Contact Time: The local or systemic effect is dependent on time of contact of MCG in oral cavity. In clinical trial chewing time of 30 minutes was considered close to ordinary use.
5. Physicochemical properties of active ingredient: Physicochemical properties of active ingredient plays very important role in release of drug from MCG. The saliva soluble ingredients will be immediately released within few minutes whereas lipid soluble drugs are released first into the gum base and then released slowly.
6. Formulation factor: Composition and amount of gum base affect rate of release of active ingredient. If lipophilic fraction of gum is increased, the release rate is decreased.
7. Changing the water solubility of the active substance will increase or delay the release. A similar effect may be obtained by changing the hydrophilic/lipophilic balance of the chewing gum formulation. The simplest way of achieving this is to increase or decrease the amount of gum base. An increase in the gum base will make the formulation more lipophilic and thus reduce the release rate of a given active substance. In principle, it is possible to manufacture products with a very low gum base content, but in practice a portion of chewing gum containing less than 20% gum base will have inferior chewing properties and may not be considered a viable formulation. Instead of changing the gum base content, it is far more effective to change the release properties by adding solubilizers to the formulation. This method enables release from the chewing gum of even highly insoluble substances, e.g. Miconazole. However using solubilizers requires specially designed gum bases as the solubilizer affect the texture of chewing gum. This may result in residual product becoming soft to an unacceptable degree after a very short period of chewing. Other methods are available for instance nicotine can be formulated as complex bound to a cation exchange resin leading to a prolonged release. This ion exchange principle could of course, also be used for other ionic substances. It is also possible to granulate the active substance with hydrophilic components, melted lipids, or to mix the active substance with a melted polymer.
8. The active substance: The release rate of an active substance depends on the solubility of the active substance in water and saliva. Highly hydrophilic substance will be almost completely released within 10 to 15 minutes. Substances with solubility in water or less than 0.1 – lg/100ml are lipophilic components of the gum base and thereby show a slow and incomplete release. Active substances may be found in the form of salts or compounds with different solubilities, e.g. pro-drugs, thus the compound offering the best properties for achieving optimal release may be selected. Chlorhexidine can serve as an example apart from pure chlorexidine, chlorhexidine is available as different salts with different solubility. A special compound or pro-drug may be obtained by formulating a complex with an active lipophilic substance, e.g. by using cyclodextrines. This will result in a compound with higher water solubility and consequently increased release. It is also possible to increase or delay the release of an active substance by changing the physical form through a variety of coating and encapsulating techniques of the substance particles. A hydrophilic or a hydrophobic coating may encapsulate the active substance. To reduce the release rate a coating with ethyl cellulose can be used.
9. The individual: For medical chewing gum as for other pharmaceutical products is an inter-patient variance. Additional to conventional pharmaceutical formulations, other interpatient variations apply for a chewing gum formulation. When the individual is chewing the gum it may be regarded as an extraction process. Consequently, the release is related to the time the gum is being chewed to the frequency and intensity by which the individual is chewing, and it depends on the amount and composition of the individual’s saliva.
PHARMACEUTICAL SIGNIFICANCE OF MEDICATED CHEWING GUMS
Prevention and cure of oral disease are obvious targets for chewing gum formulations. Chewing gum can release an active substance at a controlled rate over an extended period of time providing a prolonged local effect.
1. Sugar free chewing gum is known to be beneficial to dental health. It has been shown that use of sugar free chewing gum after meals re-elevates plaque. pH plays an important role in the development of dental caries. Therefore, in caries prevention programs, sugar-free chewing gum is recommended after meals and snacks as a supplement to tooth brushing.
2. Indications for fluoride chewing are prevention of dental caries in children in fluoride deficient areas, in adults with a high incidence of caries and in patients with xerostomia.
3. Chlorhexidine chewing gum can be used for alleviations of gingivitis, periodontitis and other oral and pharyngeal infections. It can also be used for inhibition of plaque growth and has proven valuable in oral health care of the elderly. Furthermore, chlorhexidine in a chewing gum formulation gives less staining of the teeth and is more convenient to use than a chlorhexidine mouth rinse. The chlorhexidine released by chewing is distributed evenly in the oral cavity and is present there for a prolonged time. The bitter taste of chlorhexidine can be masked quite well in achieving gum formulation.
4. Smoking cessation- Chewing gum formulation containing nicotine49, lobeline and silver acetate have been clinically tested as aids to smoking cessation. Nicotine is a natural alkaloid occurring in the leaves of tobacco plant. It is a therapeutic agent intended to help smokers break the psychological habit of smoking by reducing the nicotine withdrawal symptoms normally experienced when smoking is stopped. The formulation nicorette available as mint and classic with different flavor and dosage, is developed with ion- exchange resin, released 90% of drug after 30 min chewing (Russel et.al 1980). The release rate was controlled by the rate and vigour of chewing. Thus the patient can control the drug intake to match his needs. Increasing the pH of the mmedium in which it is dissolved can enhance nicotine absorption.
5. Clinical trials involving patients with oral candidia sis have shown that miconazole chewing gum is at least as efficient as miconazole oral gel in the treatment of fungal infections in the mouth. Furthermore, patients preferred chewing gum to oral gel due to convenience and fewer side effects.
6. Chewing gum as a drug delivery system also provides benefits to systemic drug delivery, especially if the active substance is absorbed through the buccal mucosa, fast and acute treatment, convenience, no need for water and thereby easy administration – anytime anywhere – reduced risk of gastrointestinal side effects. These benefits apply not only to the treatment of adults, but also to the treatment of children and adolescents. Systemic effect of active substances released from chewing gum can be achieved in two ways. In the “traditional” way by swallowing the active substances, or via absorption through the oral mucosa. The latter is of special interest, as buccal absorption avoids first-pass hepatic metabolism of the active substance, it could provide better bioavailability. Buccal absorption may also lead to fast onset of the action and lead directly in to systematic circulation. Chewing gum promotes buccal absorption by releasing active substances at carefully controlled rates, thus allowing for extended exposure in the oral cavity.
7. A study of pharmacokinetics of nicotine chewing gum indicated that some of the nicotine was not absorbed through route but was swallowed and underwent first-pass metabolism. It was estimated that approximately 80% of the nicotine released from the chewing gum was absorbed through buccal route.
8. Successful treatment of minor pains, headaches, pains of colds, muscular ache, etc. requires rapid absorption of therapeutic doses of active substance. Chewing gum as a drug delivery system could be beneficial in minor pain treatment, when buccal absorption results in fast onset of action and reduces the risk of gastrointestinal side effects.
9. The bioavailability of acetylsalicylic acid in a chewing gum formulation relative to an unbuffered tablet formulation has been determined. Absorption from the chewing gum formulation was shown to be faster than absorption from the tablet, and consequently, a chewing gum formulation may provide faster pain relief.
10. Several chewing gum formulations containing caffeine, guarana or chromium are available. Caffeine and guarana are central stimulating anorectic agents that have been proved to increase the metabolic rate. Moreover, they stimulate lipolysis, have a thermogenic effect (increase energy expenditure) and reduce the feeling of hunger.
11. Chromium is claimed to reduce the carving for food due to an improved blood glucose balance. Chewing gum has been proven efficient in the treatment involving instant raving and “oral habits”. Hence there is a rationale for administering weight reducing active substance in a chewing gum formulation.
12. Allergy, nausea, motion sickness, diabetes, anxiety, dyspepsia, osteoporosis, and cough and cold are all indications for which chewing gum as a drug delivery system could be beneficial.
13. Chewing gum containing antacids or mucolytics also presents advantages for patients.
14. Chewing gum as a drug delivery system offers convenience in the treatment/prevention of motion sickness and nausea. Medicated chewing gum containing dimenhydtinate for motion sickness is already on the market, however, active substances like scopolamine, metoclopramide, ondansetron and dolasetron may be candidates for a chewing gum formulation for the treatment/prevention of motion sickness and nausea.
15. Several chewing gum formulations containing calcium are available on the market. Adolescents constitute a potential target group for a calcium chewing gum as the calcium intake of young people is often very low. Calcium chewing gum with a pleasant flavour is an attractive and convenient alternative to tablets.
16. Miconazole has also been formulated as chewing gum and these formulations have been used in clinical trails.
17. As Propranolol exhibits first-pass metabolism, a chewing gum formulation was seen as a viable option to obtain buccal absorption.
IN-VITRO DRUG RELEASE TESTING APPARATUS:
Apparatus I: Number of apparatus for studying in-vitro drug release from medicated chewing gum has been developed. In 2000, European Pharmacopoeia published a monograph describing a suitable apparatus for studying the in-vitro release of drug substances from MCG.
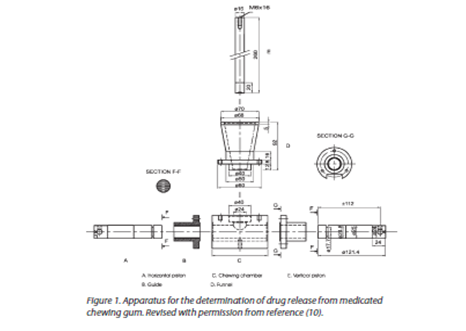
Description of apparatus:
The chewing apparatus for medicated chewing gum was adopted by Ph. Eur. in 2000 (8). Figure 1 shows the construction of the apparatus. The chewing apparatus comprises a chewing chamber, two horizontal pistons, and a third vertical piston (tongue). The vertical piston operates alternatively with the two horizontal pistons and makes sure the gum stays in the right place between chews. If necessary, it is feasible to construct the machine so that at the end of the chew the horizontal pistons rotate around their own axes in opposite directions to each other to obtain maximum chewing. The working procedure of this chewing apparatus is described in
SAFETY ASPECTS
Generally, today it is perfectly safe to chew chewing gum. Previously, hard chewing gum has caused broken teeth. Extensive chewing for a long period of time may cause painful jaws muscle, and extensive use of sugaralcohol containing chewing gum may cause diarrhea. Long term frequent chewing of gum has been reported to cause increased release of mercury vapors from dental amalgam fillings. However, medicated chewing gum does not normally require extensive chewing, or consumption to great extent. Flavors, colour etc. may cause allergic reactions. Overdosing by use of chewing gum is unlikely because a large amount of gum has to be chewed in a short period of time to achieve this. Swallowing pieces of medicated chewing gum will only cause minor release of the drug because the drug can only be released from the gum base by active chewing. As a general rule, medicated chewing gum (like other medicines) should be kept out of reach of children, if required; drug delivery may be promptly terminated by removal of the gum.
FUTURE TRENDS:
Chewing gum not only offers clinical benefits but also is an attractive, discrete and efficient drug delivery system. A few decades ago, the only treatment for some disease was surgical procedure but now more and more disease can be treated with Novel Drug Delivery Systems. Generally, it takes time for a new drug delivery system to establish itself in the market and gain acceptance by patients, however chewing gum is believed to manifest its position as a convenient and advantageous drug delivery system as it meets the high quality standards of pharmaceutical industry and can be formulated to obtain different release profiles of active substances. The potential of MCG for buccal delivery, fast onset of action and the opportunity for productline extension makes it an attractive delivery form. Reformulation of an existing product is required for patent protection, additional patient benefits and conservation of revenues.
SUMMARY AND CONCLUSION
Thus, it can be concluded that the chewing gum can be used, as a carrier for vast categories of drugs where extended release and the local action is desired. Chewing gum can be used without water, at any time. Medicated Chewing gums can produce both local effects as well as systemic effects in the oral cavity. They can be used for the purpose of taste masking of certain drugs too.
References
1. Michael J.R, Jonathan H and Michael S.R, Modified- Release Drug Delivery Technology, New York: Marcel Dekker, 2002, 419-429.
2. European Pharmacopoeia, 3rd ed.
3. British Pharmacopoeia, 3rd ed., 2001, 1778, A252.
4. Documentation on medical chewing gum, 2003, Fertin Pharma A/S
5. Advantages of medical chewing gum, 2003, Fertin Pharma A/s
6. Chewing gum as drug delivery system, 2002, Fertin Pharma A/s
7. Development of medical chewing gum, 2002, Fertin Pharma A/S.
8. Cherukuri….et al., United States Patent, 4, 753, 805 December 8, 1998.
9. Eisenstadt… et al., United States Patent, 5, 846, 557 December 8, 1998.
10. West Douglas H, United States Patent, 6, 537, 525 January 29, 1997.
11. Jinsong Hao, Paul W.S. Heng, Buccal delivery system, Drug Develop Ind Pharm., 2003, 821,852.
12. Naik Heema ,Gupta Stuti. MEDICATED CHEWING GUMS- UPDATED REVIEW. International Journal of Pharma Research and Development – Online. 2010/PUB/ARTI/VOV-2/ISSUE-8/OCT/011.









