ABOUT AUTHOR:
Mayure Vijay Kumar*, G.J.Finny, C.P.Meher
Department of pharmacology,
Maheshwara College Of Pharmacy, Chitkul(v), Isnapur “X” Road,, Patancheru, Hyderabad-A.P-502307
*mayurevijaykumar@gmail.com
ABSTRACT:
Prostaglandins are highly potent substances that are not stored but are produced as needed by cell membranes in virtually every body tissue. Different prostaglandins have been found to raise or lower blood pressure and regulate smooth muscle activity and glandular secretion. One such substance, which stimulates contraction of the uterus, is used clinically to induce labor; another has been in experimental use as a birth control agent. Prostaglandins also control the substances involved in the transmission of nerve impulses, participate in the body's defenses against infection, and regulate the rate of metabolism in various tissues. Several prostaglandins have been shown to induce fever, possibly by participating in the temperature-regulating mechanisms in the hypothalamus; they also play a part in causing inflammation. The fact that aspirin and other nonsteroidal anti-inflammatory drugs have been shown to inhibit prostaglandin synthesis may account for their usefulness in reducing fever and inflammation. Many naturally occurring prostaglandins as well as many artificial forms have been synthesized in the laboratory. The following article put forth the detailed information about the prostaglandins.
REFERENCE ID: PHARMATUTOR-ART-1716
INTRODUCTION:
A prostaglandin is any member of a group of lipid compounds that are derived enzymatically from fatty acids and have important functions in the animal body. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are mediators and have a variety of strong physiological effects, such as regulating the contraction and relaxation of smooth muscle tissue. Prostaglandins are not endocrine hormones, but autocrine or paracrine, which are locally acting messenger molecules. They differ from hormones in that they are not produced at a discrete site but in many places throughout the human body. Also, their target cells are present in the immediate vicinity of the site of their secretion (of which there are many).The prostaglandins, together with the thromboxanes and prostacyclins, form the prostanoid class of fatty acid derivatives, a subclass of eicosanoids[1].
BIOCHEMISTRY: Biosynthesis

Biosynthesis of eicosanoids:
Prostaglandins are found in most tissues and organs. They are produced by almost all nucleated cells. They are autocrine and paracrine lipid mediators that act upon platelets, endothelium, uterine and mast cells. They are synthesized in the cell from the essential fatty acids (EFAs). An intermediate arachidonic acid is created from diacylglycerol via phospholipase-A2, then brought to either the cyclooxygenase pathway or the lipoxygenase pathway to form either prostaglandin and thromboxane or leukotriene respectively. The cyclooxygenase pathway produces thromboxane, prostacyclin and prostaglandin D, E and F. Alternatively, the lipoxygenase enzyme pathway is active in leukocytes and in macrophages and synthesizes leukotrienes.
RELEASE OF PROSTAGLANDINS FROM THE CELL:
Prostaglandins were originally believed to leave the cells via passive diffusion because of their high lipophilicity. The discovery of the prostaglandin transporter (PGT, SLCO2A1), which mediates the cellular uptake of prostaglandin, demonstrated that diffusion alone cannot explain the penetration of prostaglandin through the cellular membrane. The release of prostaglandin has now also been shown to be mediated by a specific transporter, namely the multidrug resistance protein 4 (MRP4, ABCC4), a member of the ATP-binding cassette transporter superfamily. Whether MRP4 is the only transporter releasing prostaglandins from the cells is still unclear.
Cyclooxygenases:
Prostaglandins are produced following the sequential oxidation of AA, DGLA or EPA by cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin synthases. The classic dogma is as follows:
- COX-1 is responsible for the baseline levels of prostaglandins.
- COX-2 produces prostaglandins through stimulation.
However, while COX-1 and COX-2 are both located in the blood vessels, stomach and the kidneys, prostaglandin levels are increased by COX-2 in scenarios of inflammation.
Prostaglandin E synthase:
Prostaglandin E2 (PGE2) is generated from the action of prostaglandin E synthases on prostaglandin H2 (PGH2). Several prostaglandin E synthases have been identified. To date, microsomal prostaglandin E synthase-1 emerges as a key enzyme in the formation of PGE2.
Other terminal prostaglandin synthases:
Terminal prostaglandin synthases have been identified that are responsible for the formation of other prostaglandins. For example, hematopoietic and lipocalin prostaglandin D synthases (hPGDS and lPGDS) are responsible for the formation of PGD2 from PGH2. Similarly, prostacyclin (PGI2) synthase (PGIS) converts PGH2 into PGI2. A thromboxane synthase (TxAS) has also been identified. Prostaglandin-F synthase (PGFS) catalyzes the formation of 9α, 11β-PGF2α,β from PGD2 and PGF2α from PGH2 in the presence of NADPH. This enzyme has recently been crystallized in complex with PGD2[2] and bimatoprost[3] (a synthetic analogue of PGF2α). Prostaglandins are potent but have a short half-life before being inactivated and excreted. Therefore, they send only paracrine (locally active) or autocrine (acting on the same cell from which it is synthesized) signals.
|
TYPES: The following is a comparison of different types of prostaglandin, prostacyclin I2 (PGI2), prostaglandin E2 (PGE2), and prostaglandin F2α (PGF2α).
|
ROLE IN PHARMACOLOGY:
[adsense:468x15:2204050025]
Inhibition: Prostaglandin antagonist and Mechanism of action of aspirin
Examples of prostaglandin antagonists are:
- NSAIDs (inhibit cyclooxygenase)
- Corticosteroids (inhibit phospholipase A2 production)
- COX-2 selective inhibitors or coxibs
- Cyclopentenone prostaglandins may play a role in inhibiting inflammation.
Clinical uses:
Synthetic prostaglandins are used:
- To induce childbirth (parturition) or abortion (PGE2 or PGF2, with or without mifepristone a progesterone antagonist);
- To prevent closure of patent ductus arteriosus in newborns with particular cyanotic hear defects (PGE1)
- To prevent and treat peptic ulcers (PGE)
- As a vasodilator in severe Raynaud's phenomenon or ischemia of a limb
- In pulmonary hypertension
- In treatment of glaucoma (as in bimatoprost ophthalmic solution, a synthetic prostamide analog with ocular hypotensive activity)
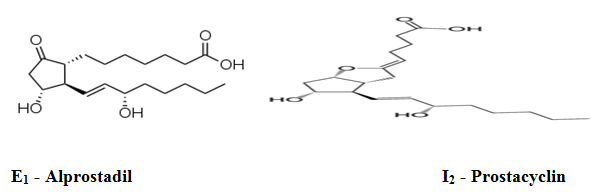
- To treat erectile dysfunction or in penile rehabilitation following surgery (PGE1 as alprostadil).[7]
- To treat egg binding in small birds[8]
- As an ingredient in eyelash and eyebrow growth beauty products due to side effects associated with increased hair growth.
BALL AND STICK MODEL OF PROSTACYCLIN (PROSTAGLANDINS):
Synthetic prostacyclin analogues (iloprost, cisaprost) are used intravenously, subcutaneously or by inhalation:
- as a vasodilator in severe Raynaud's phenomenon or ischemia of a limb;
- in pulmonary hypertension.
- in primary pulmonary hypertension (PPH)
Its production is inhibited indirectly by NSAIDs, which inhibit the cyclooxygenase enzymes COX1 and COX2. These convert arachidonic acid to prostaglandin H2 (PGH2), the immediate precursor of prostacyclin. Since thromboxane (an eicosanoid stimulator of platelet aggregation) is also downstream of COX enzymes, one might think that the effect of NSAIDs would act to balance. However, prostacyclin concentrations recover much faster than thromboxane levels, so aspirin administration initially has little to no effect but eventually prevents platelet aggregation (the effect of prostaglandins predominates as they are regenerated). This is explained by understanding the cells that produce each molecule, TXA2 and PGI2. Since PGI2 is primarily produced in a nucleated endothelial cell, the COX inhibition by NSAID can be overcome with time by increased COX gene activation and subsequent production of more COX enzymes to catalyze the formation of PGI2. In contrast, TXA2 is released primarily by anucleated platelets, which are unable to respond to NSAID COX inhibition with additional transcription of the COX gene because they lack DNA material necessary to perform such a task. This allows NSAIDs to result in PGI2 dominance that promotes circulation and retards thrombosis. In patients with pulmonary hypertension, inhaled epoprostenol reduces pulmonary pressure, and improves right ventricular stroke work in patients undergoing cardiac surgery. A dose of 60 µg is hemodynamically safe, and its effect is completely reversed after 25 minutes. No evidence of platelet dysfunction or an increase in surgical bleeding after administration of inhaled epoprostenol has been found.[9]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PROSTAGLANDIN ANTAGONIST:
A Prostaglandin antagonist is a hormone antagonist acting upon prostaglandin. NSAIDs inhibit cyclooxygenase and reduce prostaglandin synthesis. Corticosteroids inhibit phospholipase A2 production by boosting production of lipocortin, an inhibitor protein. Relatively new drugs, known as COX-2 selective inhibitors or coxibs, are used as specific inhibitors of COX-2. The development of these drugs allowed the circumvention of the negative gastrointestinal effects while effectively reducing inflammation[12].
TYPES OF PROSTAGLANDINS:
Prostaglandins F2α:

Prostaglandin F2α (PGF2α in prostanoid nomenclature), pharmaceutically termed dinoprost (INN), is a naturally occurring prostaglandin used in medicine to induce labor and as an abortifacient. In domestic mammals, it is produced by the uterus when stimulated by oxytocin, in the event that there has been no implantation during the follicular phase. It acts on the corpus luteum to cause luteolysis, forming a corpus albicans and stopping the production of progesterone. Action of PGF2α is dependent on the number of receptors on the corpus luteum membrane. The PGF2α isoform 8-iso-PGF2α was found in significantly increased amounts in patients with endometriosis, thus being a potential causative link in endometriosis-associated oxidative stress.[11]
Side effects:symptoms mimic dysmenorrhea because of myometrial contraction & uterine ischemia.
Prostaglandin I2:
PGI2 is primarily synthesized in macrovascular endothelial cells. It has been shown to induce vasodilation, inhibit platelet aggregation, as well as demonstrate fibrinolytic and cytoprotective activity. PGI2 binding to the G-protein coupled IP1 receptor, is believed to be responsible for the inhibition of platelet aggregation and vasodilation activity. PGI2 greatly increases cAMP concentrations, it is 30 times more active than PGE1, and 10 times more active than PGD2; however , it is a relatively unstable eicosanoid, with a half-life of about 2 minutes under physiological conditions, Analogues such as Iloprost have been developed with superior stability and similar potency for IP receptors[14].
Prostaglandin E2:
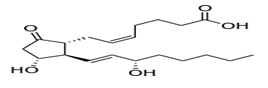
The naturally occurring prostaglandin E2 (PGE2) is known in medicine as dinoprostone. It has important effects in labour (softens cervix and causes uterine contraction) and also stimulates osteoblasts to release factors that stimulate bone resorption by osteoclasts. PGE2 is also the prostaglandin that ultimately induces fever.
It is sold under the trade name of Cervidil (by Forest Laboratories, Inc.), Prostin E2 (by Pfizer Inc.), Propess (by Ferring Pharmaceuticals) andGlandin (by Nabiqasim Pharmaceuticals Pakistan) as a vaginal suppository, to prepare the cervix for labour; it is used to induce labour.
Like other prostaglandins, dinoprostone can be used as an abortifacient. It is a direct vasodilator, relaxing smooth muscles, and it inhibits the release of noradrenaline from sympathetic nerve terminals. It does not inhibit platelet aggregation, where PGI2 does.It works by binding and activating the prostaglandin E2 receptor. Up-regulation of PGE2 has been implicated as a possible etiology of nail clubbing.
Precautions : uterine scar tissues; asthma; low blood pressure; heart disease; adrenal problems; anemia; diabetes; glaucoma; icterus (jaundice); multiparity (5 pregnancies); heart, lung or liver disease[10].
PGE2 & PGI2:
•PGE2 & PGI2 maintain uterine quiescence by increasing C AMP signaling. They stimulate adenylcyclase activity in myometrium at 32 to 35wks leading to relaxation of vascular smooth muscle, vasodilatation.
•At 39 to 40 wks, regional myometrial contractions in fundus occurs after initiation of parturition.
•PGE2 synthesis in renal medulla is markedly increased in late pregnancy and it acts as natriuretic[13].
Types of prostaglandins commercially available:
• Two forms of PGE2 are available commercially. The first is Prostaglandin E2 (dinoprostone) formulated as gel and is placed inside the cervix but not above the internal os. Prostaglandin E2 (dinoprostone) is licensed for the use of labour induction in the cases of viable pregnancies.The application ( 3g gel / 0.5 mg dinoprostone) can be repeated in 6 hrs, not to exceed 3 doses in 24 hrs.
• The second form is 10 mg of dinoprostone embedded in a mesh and is placed in the posterior fornix of vagina, this allows for control release of dinoprostone over 12 hrs, after which it is removed.
• Prostaglandin E1 analog ( misoprostol) available in tablet form for induction of labor was described recently in a series of articles. This is a synthetic prostaglandin, which is marketed as an antinuclear agent under the trade name cytotec. 25 or 50 micro g placed in the posterior fornix, has been shown in several studies to be quite effective in inducing cervical ripening and initiation of abortion or labor. The application of medication can be repeated every 4 -6 hrs up to 5 doses.
The major risk of above prostaglandin preparation is uterine polysystoly, hyper stimulation, meconium stained liquor and fetal distress. The women and fetus must be monitored for contractions, fetal wellbeing and changes in Bishop score[13].
Administration routes of prostaglandins:
• By the mid 1980s prostaglandins had become established as the most effective pharmacological agents for inducing abortions and labor when the cervix is unripe.
• A variety of administration routes had been employed during the preceding years, including oral, intravenous, sublingual, rectal, intra amniotic, extra amniotic, intra cervical, and vaginal administration.
• The vaginal route is found to be the most acceptable, providing good efficacy and acceptability for the parturient and is now the preferred method of choice.
Uses of prostaglandins:
For first trimester (upto 12 weeks) abortions:
• For first trimester abortion prostaglandins can be used in combination. That is with mifepristone . Mifepristone 600mg orally, followed by a misoprostol 800 micro g vaginally a day later.
• Or mifepristone 600 mg followed by 400 micro g of misoprostol orally can be used.
• Or lower dose mifepristone 200 mg followed by 800micro g of misoprostol , 24 to 72 hrs later vaginally can also be used.
• Repeated doses of misoprostol can be used for delayed expulsions.
• Other regimes are Tomoxifen 20 mg daily for 4 days followed by misoprostol 800 micro g vaginally, second dose 24 hrs later if needed. Or Methotrexate 25 to 50 mg orally or 75 mg IM, followed by 800 micro g vaginally after a week.
• Misoprostol 800 micro g daily for 3 days can be used in late first trimester.
• In very early gestation single vaginal dose of 800 micro g, or multiple doses within 24 hrs are enough.
• Infants born to mothers exposed to misoprostol, may have abnormal vascular development, mobius's syndrome, congenital facial paralysis with or without limb defects, equinovarus, arthogryposis etc.
• Women with medical abortions experience more bleeding and cramping than spontaneous abortions.
For second trimester (12 to 20 weeks) abortions:
• PGE2 (dinoprostone) can be used in the form of 20 mg suppositories kept in the post vaginal fornix.
Side effects: nausea, vomiting, fever, diarrhoea etc.
• Or PGE1 ( misoprostol) can be used 600 micro g vaginally followed by 400 micro g every 4 hrly.
• But even after usage of prostaglandins, 2% of women may require curettage for retained placenta[13].
PROSTAGLANDIN IN LABOUR (induction of labour):
Prostaglandin is naturally occurring hormone that prepares your body for labour. A synthetic version has been developed to mimic the effect of the hormone. This is inserted into vagina, usually in the form of a gel. It can also be inserted in the form of a pessary which slowly releases the prostaglandins over 12-24h. when the prostaglandins is in place, you will be advised to lie down and rest for at least 30min. once the prostaglandins has been inserted you will need to remain in hospital. When the prostaglandin takes effect, your cervix will soften and open. if the gel is used, you may require one, two, or three doses (given every 6-8h). when the cervix is soft and open, your body is prepared for labour. The next steps will vary from woman to woman- some might require an ARM to break their waters’, whereas this might happen naturally for other women. Some women might require oxytocin to stimulate the concentrations[15].
Effects of Aspirin and other Pain Killers:
When prostaglandins induce inflammation, pain, and fever, what comes to mind but aspirin. Aspirin blocks an enzyme called cyclooxygenase, COX-1 and COX-2, which is involved with the ring closure and addition of oxygen to arachidonic acid converting to prostaglandins. The acetyl group on aspirin is hydrolzed and then bonded to the alcohol group of serine as an ester. This has the effect of blocking the channel in the enzyme and arachidonic can not enter the active site of the enzyme. By inhibiting or blocking this enzyme, the synthesis of prostaglandins is blocked, which in turn relives some of the effects of pain and fever. Aspirin is also thought to inhibit the prostaglandin synthesis involved with unwanted blood clotting in coronary heart disease. At the same time an injury while taking aspirin may cause more extensive bleeding[16]. Prostaglandins influence every organ in the body and also activate as a response to tissue damage; as white blood cells move to a site to decrease tissue destruction, they produce these substances. Prostaglandins are directly responsible for tissue inflammation, fever and pain. Certain types of prostaglandins also promote sodium retention and increase cortisol production in the body[17].
PROSTAGLANDINS DEFICIENCY:
Schizophrenia as a prostaglandin deficiency disease:
Evidence that schizophrenia may be a prostaglandin deficiency disease comes from three main sources:
1) All effective antischizophrenic drugs stimulate prolactin secretion and prolactin is a potent stimulator of prostaglandin synthesis.
2) schizophrenics are resistant to pain and inflammation and are free of rheumatoid arthritis and there is increasing evidence that prostaglandins play important roles in pain, inflammation, and rheumatoid arthritis.
3) High doses of drugs recently shown to be prostaglandin antagonists cause schizophrenia-like syndromes. The hypothesis is not necessarily inconsistent with current transmitter theories of schizophrenia since prostaglandins modify transmitter secretion and action. It does indicate radically new approaches to investigation, treatment, and drug design not suggested by the transmitter concepts[18]
The Enteropathy of prostaglandin deficiency:
Small intestinal ulcers are frequent complications of therapy with nonsteroidal anti-inflammatory drugs (NSAIDs). Eicosanoids and metabolites were measured by isotope dilution with mass spectrometry. cDNA was obtained by reverse transcription and sequenced following amplification with RT-PCR. We investigated the cause of chronic recurrent small intestinal ulcers, small bowel perforations, and gastrointestinal blood loss in a 45-year-old man who was not taking any cyclooxygenase inhibitor. Prostaglandin metabolites in urine were significantly depressed. Serum thromboxane B2 (TxB2) production was 4.6% of normal controls (P<0.006), and serum 12-HETE was 1.3% of controls (P<0.005). Optical platelet aggregation with simultaneous monitoring of ATP release demonstrated absent granule secretion in response to ADP and a blunted aggregation response to ADP and collagen, but normal response to arachidonic acid (AA). LTB4 biosynthesis by ionophore-activated leukocytes was only 3% of controls, and urinary LTE4 was undetectable. These findings suggested deficient AA release from membrane phospholipids by cytosolic phospholipase A2-alpha (cPLA2-alpha), which regulates cyclooxygenase- and lipoxygenase-mediated eicosanoid production by catalyzing the release of their substrate, AA. Sequencing of cPLA2-alpha cDNA demonstrated two heterozygous nonsynonymous single-base-pair mutations: Ser111Pro (S111P) and Arg485His (R485H), As well as a known single nucleotide polymorphism (SNP), Lys651Arg (K651R). Characterization of this cPLA2-alpha deficiency provides support for the importance of prostaglandins in protecting small intestinal integrity and indicates that loss of prostaglandin biosynthesis is sufficient to produce small intestinal ulcers [19].
Effect of bisenoic prostaglandins on the uterine vasculature of the nonpregnant sheep:
The effects of the bisenoic prostaglandins on the uterine vasculature and uterine contractile activity have been evaluated in an unanesthetized chronically catheterized nonpregnant sheep preparation. Changes in uterine blood flow were monitored with electromagnetic flow probes while uterine contractile activity and tone were determined via an intra-uterine balloon connected to a pressure transducer. Prostaglandins A2, D2, E2, and prostacyclin (PGI2) were all found to be vasodilators. PGD2 and PGI2 were much more potent than PGA2 and PGE2 in dilating the uterine vasculature. The prostacyclin breakdown product 6-keto PGF1 alpha, PGF2 alpha, thromboxane B2, and the endoperoxide analogues U44069 and U46619 produced vasoconstriction of the uterine vasculature. Prostaglandins A2, D2 and F2 alpha increased while PGI2 decreased uterine contractile activity. PGF2 alpha also increased uterine tone suggesting that a portion of its vasoconstrictor activity may be due to mechanical compression of the uterine vasculature [20].
The putative role of prostaglandins in surgical miosis.
Most clinical studies demonstrate a small (0.5-1.0 mm) but significant reduction of surgical miosis bycyclooxygenase inhibitors (COIs), such as indomethacin, flurbiprofen, or suprofen. However, other studies failed to show significant inhibition by some of these agents. While this inhibition may provide a clinically useful advantage in some cases, it does not help us clarify the mediators and mechanism of the miotic response. The inhibitory effect of high doses of COIs on surgical miosis has generally been explained on the basis of inhibition of PG synthesis. However, COIs do not selectively inhibit the synthesis of PGs; they inhibit the synthesis of all cyclooxygenase products, including prostacyclin andthromboxanes. The reduction of the miotic response could also have resulted, in part, from inhibition of the lipoxygenase pathway of arachidonic acid metabolism. The effect of COIs on unrelated biochemical processes that influence the contractile responses of the iris sphincter may also have contributed to the inhibition of surgically induced miosis. In short, the mechanism by which COIs reduce miosis remains unidentified. If one assumes that PGs are the only mediators of this miosis, one must also assume that failure of COIs to block surgical miosis is due to incomplete inhibition of cyclooxygenase activity in the involved ocular tissues, even though the COIs are applied repeatedly to the eye prior to surgery. However, a more likely explanation is that other mediators are involved in themiotic response, especially since no PG has yet been shown to produce a dose-dependent mioticresponse leading to full sphincter contraction in any primate (see Miranda and Bito, 1989). Whileacetylcholine is the best-established agonist of iris sphincter contraction, surgical miosis occurs even after pretreatment with high doses of potent anti-cholinergic agents. This atropine-resistant component of the surgically induced miosis may be mediated by a number of autocoids. Several autacoids, other than PGs, have been shown to be potent miotic agents in some animals. LTC4 and LTD4, which are potent miotics in cats, have not yet been investigated for their possible role in surgical miosis in primates. Several neuropeptides are potent miotics in rabbits, although none of them has yet been found to have similar effects on human irides. Because sensory neuronal involvement in trauma-induced miosis has been demonstrated in many species, including humans (see Stjernschantz and Bito, 1989; also Unger, 1989), a search for a neuropeptide that has miotic properties in humansshould continue. Much more work must be done before the mechanism of surgical miosis can be clarified [21]
Inhibition of human platelet aggregation by prostaglandins:
IC50values (µg/ml)Aggregating Agent
|
|
16,16-diMe-PGE2(3µg/ml) |
Wy-17,186(3µg/ml) |
ADP(1µM) |
|
PGD2 |
0.06 |
0.07 |
0.01 |
|
PGE1 |
0.02 |
0.06 |
0.01 |
|
PGE2 |
0.20 |
0.80 |
10 |
|
15-Me-15(R)-PGE2 |
50 |
45 |
>100 |
|
PGF2α |
2.5 |
4.0 |
>100 |
Results are mean values of triplicate determinations [22].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHYSIOLOGICAL ROLES OF PROSTAGLANDINS AND OTHER EICOSANOIDS IN INVERTEBRATES:
Control of hatching:
In the barnacle Balanus balanoides, full egg-laying involves passing eggs along oviducts into ovi sacs produced by oviducal glands. Fully formed egg masses are finally released into the mantle cavity, where they remain until hatching which corresponds with spring algal blooms (Crisp, 1962; Clare et al., 1985). The synchrony offspring bloom and egg hatching could be related to a component in the nutrition of adult barnacles. However, Crisp and Spenser (1958) showed that seawater extracts of unfed and fed adults were equally effective in inducing hatching. They proposed abarnacle hatching substance, endogenously produced by adults, and showed that the substance acts upon the musculature of mature embryos, not on the egg case. The hatching substance appeared to be a PG (Clare et al., 1982, 1985). The substance is extractable in a system optimized for PGs, it behaves like a PG on thin layer chromatography, and extracts of the dried cortex of a commercial source of PG (the gorgonian Plexura homomalla) acted biologically and chemically like barnacle hatching substance. Extracts made in the presence of aspirina PG-synthetase inhibitor in mammals did not induce hatching. Clare et al. (1985) concluded that barnacle hatching substance is either a PG or a PG-like compound. Subsequent work underscores the importance of rigorouschemical methodologies in indentification of biologically active compounds. Holland et al. (1985) extracted 50 kg of barnacles, then processed the extracts through four sequential systems of thin layer chromatography. The active compound was detected by bioassay a teach stage. The purified compound was denvatized for gas chromatography-mass spectroscopy (GC-MS), which yielded a single major OC peak. Mass spectra of derivatized hatching factor and hydrogenated derivatized hatching factor were consistent, not with a PG, but with another eicosanoid, 10,1 1,12-trihydroxy-5,8,l 1,17 eicosatetraenoic acid This compound is probably a lipoxygenase derivative ofC20:5n3, an abundantly available fatty acid in marine invertebrates and also the precursor of the 3-series PGs [23].
Oocyte maturation in starfish:
Starfish oocytes develop to the first meiotic prophase, then await the spawning period. Maturation, or meiosis reinitiation, is induced by a hormone produced and released by the follicle cells surrounding the oocytes, 1-methyladenine. Once stimulated by the hormone, the oocytes complete the developmental path leading to fertilizable cells. Arachidonic and eicosapentaenoic acids also induce oocyte maturation in three species of starfish: Asterias rubens, Marthosterius glacial is, and Luidia ciliaris (Meijer et a!., 1984). The PUFA-induced maturation is specific to these two fatty acids because 35 other fatty acids, ranging from C4:0 to C24: 1 and including satu rated, monounsaturated, and polyunsaturated fatty acids, did not induce maturation. The maturation effect is dependent upon extracellular calcium and occurs at physio logical concentrations (i.e., 50% maturation dose = 0.65 zMarachidonic acid). The fatty acids stimulate the complete maturation program, including germinal vesicle breakdown, fertilization, and development into normal larvae. Fatty acids endogenous to the oocytes are able to stimulate maturation because addition of phospholipase A2, an enzyme that hydrolyses the fatty acid from the beta-carbon of phospholipids, also stimulated maturation. The phospholipase effect was calcium-dependent, and specific because phospholipases C and D did not bring on maturation. The hormone effect probably proceeds through release and metabolism of PUFAs.Two phospholipase A2 inhibitors in mammals, quinacrine and bromophenacyl bromide, inhibit hormone-stimulated maturation, which can be overcome by increasing 1-methyl adenine concentrations. Five PGs did not stimulate maturation, and three cycbooxygenase inhibitors-acetylsalicylic acid, indomethacin, and tolazoline-did not inhibit maturation. On the other hand, three lipoxygenase inhibitors in mammals quercetin, eicosatetraynoic acid and butylated hydroxytoluene did inhibit hormone-induced maturation. Four products of lipoxygenase metabolism of arachidonic acid, 12- and 15-hydroxyeicosatetranoic acids (HETE) and their corresponding hydroperoxy eicosatetraenoic acids (HPETE) stimulated maturation. Oocytes convert radioactive arachidonic acid into HETEs (Meijer et al., 1986a). Conversion of arachidonic acid does not occur in the absence of calcium, nor are oocytes stimulated to maturation. Following incubation with radioactive arachidonic acid, fractions with chromatographic behavior of HETEs were recovered and found to stimulate oocyte maturation. The lipoxygenase inhibitor eicosatetray inhibited both conversion of arachidonic acid and stimulation of oocytes. It would appear, then, that l-methyladenine acts by release of PUFA, followed by conversion to a biologically active HETE, which induces maturation ofthe oocytes. Injection studies suggested that 12- and 15-HETE and corresponding HPETEs stimulated oocyte maturation (Meijer et al., 1984). Upon re-evaluation, it was found that the tested compounds were contaminated with 5% of 8-HETE, the active compound in maturation (Meijer et al., 1986a). Meijer et al. (1987) showed that (8R)-HETE, but not (8S)-HETE, is produced by starfish oocytes. The R isomer is the only active compound when tested in pure form, and other lipoxygenase products, including other HETEs and leukotrienes are not active.A survey ofeight starfish species shows that while the hormone 1-methyladenine stimulates maturation in all species, the stimulatory effect ofarachidonic acid and 8-HETE occurs in only three ofthem (Meijer et al., l986b). Species differences in response to various eicosanoids also have been observed in various physiological settings in mammals. At this early period of appreciating the possible physiological activities of these compounds in invertebrates systems, species differences underscore the hazards inherent in forming generalizations[23].
Cercaria!penetration of skin:
Eggs of the blood fluke Schistosoma mansoni leave their mammalian hosts in urine or feces, and continue larval development in snails. Free-swimming larvae called cercariae reinfect mammalian hosts by burrowing through the skin or by ingestion with drinking water (Storer and Usinger, 1965). It has been known for a number of years that skin surface lipids stimulate cercarial penetration of animal membranes (Stirewalt, 1971). Among the skin surface lipids, free fatty acids, especially polyunsaturated fatty acids, appeared to be most efficacious in stimulating penetration (Austin et al., 1972). Salafsky et al. (l984a) looked at the effect of certain fatty acids on two cercanal behaviors in vitro, namely cessation of swimming and initiation of penetration. Their results show that certain PUFAs attracted cercariae to the center of their test membranes while monounsaturated fatty acids did not. A few fatty acids gave intermediate results because two monounsaturated fatty acids were as stimulatory as the PUFAs, and two other monounsaturates were less stimulatory than the PUFAs but were clearly more stimulatory than controls. Cyclooxygenase metabolites, rather than the PUFAs per se, may alter cercarial behavior. Two inhibitors of cycbooxygenase ibuprofen and, to a lesser degree, aspirin inhibited cercarial response to PUFA. 13-Azaprostanoic acid, thought to specifically antagonize the platelet thromboxane/endoperoxide receptor in mammals, was also inhibitory. PUFAs and certain of their metabolites may affect cercarial penetration as well as modify behaviors that precede penetration. When Salafsky et al. (l984b) compared cercarial penetration into skin membranes prepared from essential fatty acid (EFA) deficient and EFA replete adult rats, they found about three times less penetration in the preparations from EFA deficient rats. Again, the inhibition may be related to formation of eicosanoids. Interperitoneal injections of ibuprofen led to a time-dependent accumulation of the drug in the skin of EFA replete rats. Cercarial penetration of the drug-treated skin was reduced. The percent inhibition increased with increasing amount of ibuprofen accumulated in the skin, up to a maximum inhibition of about 84%. When cercariae were incubated with radioactive linoleic acid, radioactivity could be recovered in high-pressure liquid chromatography fractions that eluted with PGE2, POD2, LTC4, LTB4 and 5-HETE. These data suggest that cycbooxygenase and lipoxygenase systems function within the cercariae. Radioimmunoassays of extracts from cercariae incubated with linoleic acid were also consistent with these products. Fusco et al. (1985) concluded that formation of eicosanoids is an essential step in penetration of human skin by cercariae of Schistosoma mansoni. If this can be supported by further work, it may present a rather interesting situation in which the PUFA substrate provided by a vertebrate host is metabolized into biologically active eicosanoids by a parasite. It is not yet known how the eicosanoids alter the behavior of the cercariae or facilitate penetration of mammalian skin. Fusco et al. (1985) suggest that vasodilation, which is induced by certain PGs, may help the parasite find and infiltrate the blood system. It would appear that the eicosanoids, in this mode, would be usurped by the parasites to alter the host physiology. In this case, the finding by Rumjanek and Simpson (1980) that adult worms do not synthesize PGE or PGF may be appreciated in terms ofhost physiology. On the other hand, the behavioral effects of cessation of swimming and initiation of penetration (Salafsky et al., 1984a), also induced by skin lipids, suggest a direct effect on the cercariae. Sponge cell aggregation Rich et al. (1984) suggest that the calcium dependent aggregation of marine sponge cells of Microcione prolifera is stimulated by leukotriene B4 (LTB4). LTB4 induced rapid cell aggregation in a dose-dependent way at 0.2 and 1.2 @tMtreatments. The effect appears to be specific for LTB4 because eight PGs of A, B, D, E, and F series and eight lipoxygenase products failed to induce aggregation. The calcium ionophore A23 187 and the species-specific aggregation factor (MAF) stimulate cell aggregation. The aggregating effects of these compounds can be inhibited by cyclooxygenase inhibitors including nordihydroquaiaretic acid and indomethicin, which also interfere with calcium flux. These data show that those agents which inhibit calcium flux also inhibit aggregation while those that promote calcium movement also promote aggregation. Interpretation is difficult because while a specific lipoxygenase product promotes aggregation, inhibitors of cycbooxygenase metabolism inhibit it. Perhaps both pathways are involved in cell aggregation, with LTB4 stimulating PG formation, which then acts in concert with the LTB4 [23].
Egg-laying behavior in crickets:
The roles of PGs in insect reproduction were reviewed by Stanley-Samuelson and Loher (1986), from which the following summary is drawn. PGs were detected in extracts of various tissues from over a dozen species of insects. The most well understood physiological role of PGs is releasing egg-laying behavior in the field cricket Teleogryllus commodus. Adult females undergo sexual maturation, during which the abdomen becomes filled with hundreds of mature eggs. Certain behaviors that are likely to bring females into contact with males also develop. Insemination is achieved by transfer of a spermatophore to the genital organ of a female from where its contents migrate into the female's spermathecae. Cycbooxygenase activity is associated with the spermatophore contents, and once in the spermathecae of newly mated females, arachidonic acid is converted into PG. It is not known how the PG formed in the spermatheca releases egg-laying behaviour, but increases in spermathecal and hemolymph PG titer after mating suggest that the PG acts at some site distant from the source. The observations that POE2 does not stimulate contraction of oviduct muscles in T. commodus (Loher, 1984) nor in a cockroach (Cook et al., 1984) and that ovi position behavior is a complex activity directed by the central, rather than peripheral, nervous system (Loher, 1984) support the hypothesis that the PGs function at the level of the central nervous system. Using egg-laying to assay structure-function relationships among a range of eicosanoids, Stanley-Samuelson et al. (1986) found that highest egg-laying activity was associated with E-series PGs. The A-,B-,D- and F-series induced zero to intermediate egg-laying. Structures that departed from the basic PG structure, represented by 15-HETE and prostacyclin, were inactive. The 2-series PGs were more active than their 1-series analogues; hence, there may be a biological specificity for POE2 in releasing egg-laying behavior in that particular cricket species. Highest egg-laying activity was induced by l5-keto-PGE2. In mammalian systems, this compound is formed by the action of prostaglandin dehydrogenase, located mainly in lungs, but also in liver and kidney. Biologically active PGE is rapidly cleared from the circulation of mammals by the activity of this enzyme. The observation that a biologically inactive compound, in the usual mammal assays, was associated with the greatest increase in egg-laying behavior marks a potentially important point in comparative physiology. Several features of the biology of eicosanoids appear to uniformly occur in the vertebrate and invertebrate systems as understood to date. For example, many compounds that inhibit the action of cycbooxygenase in mammals similarly inhibit the activity in invertebrates. On the other hand, as shown here, while the mammalian background will be important and useful in work on invertebrate systems, fundamental differences are to be expected [23].
INTERRUPTION OF EARLY PREGNANCY:
During pregnancy the uterus is maintained in a quiescent state by the secretion of progesterone. Antigestagens antagonize the biological action of progesterone by binding to the nuclear receptor in the target organs. Administration of the antigestagen mifepristone to women induces bleeding during the luteal phase and in early pregnancy by releasing endogenous prostaglandins from the endometrium or decidua. In addition, the sensitivity of the myometrium to exogenous prostaglandins is markedly increased. Although mifepristone will induce bleeding in the majority of women in early pregnancy, the incidence of incomplete abortion or ongoing pregnancies increases with increasing gestational age and is too high to be clinically useful as an agent for therapeutic abortion. However, a single dose of mifepristone (400-600 mg) followed by a vaginal pessary of a prostaglandin analogue (0.5-1.0 mg), gemeprost, induced complete abortion in 95 of 100 women of gestational age less than 42 days (less than or equal to 56 days amenorrhoea). The incidence of diarrhoea (15%) and abdominal pain requiring opiate analgesia (10%) was much lower than when abortion was induced with prostaglandin alone. Vaginal bleeding continued for 13.8 +/- 0.8 days after administration of the prostaglandin. A combination of an antigestagen with a small dose of a prostaglandin analogue is an effective alternative to vacuum aspiration for the therapeutic termination of early pregnancy [24].
STIMULATION OF HUMAN PLATELETS BY PROSTAGLANDINS:

CONCLUSION:
There are currently ten known prostaglandin receptors on various cell types. Prostaglandins ligate a sub-family of cell surface seven-transmembrane receptors, G-protein-coupled receptors. These receptors are termed DP1-2, EP1-4, FP, IP1-2, and TP, corresponding to the receptor that ligates the corresponding prostaglandin (e.g., DP1-2 receptors bind to PGD2). The diversity of receptors means that prostaglandins act on an array of cells and have a wide variety of effects such as: cause constriction or dilation in vascular smooth muscle cells,cause aggregation or disaggregation of platelets, sensitize spinal neurons to pain, induce labor, decrease intraocular pressure, regulate inflammatory mediation, regulate calcium movement, control hormone regulation, control cell growth, acts on thermoregulatory center of hypothalamus to produce fever, acts on mesangial cells in the glomerulus of the kidney to increase glomerular filtration rate, acts on parietal cells in the stomach wall to inhibit acid secretion.
REFERENCE:
1) Nelson, Randy F. (2005). An introduction to behavioral endocrinology (3rd ed.). Sunderland, Mass: Sinauer Associates. pp. 100.
2) Komoto J, Yamada T, Watanabe K, Takusagawa F (2004). "Crystal structure of human Prostaglandin-F synthase (AKR1C3)". Biochemistry 43 (8): 2188–98.
3) Komoto J, Yamada T, Watanabe K, Woodward D, Takusagawa F (2006). "Prostaglandin F2alpha formation from prostaglandin H2 by Prostaglandin-F synthase (PGFS): crystal structure of PGFS containing bimatoprost". Biochemistry 45 (7): 1987–96.
4) Rang, H. P. (2003). Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. pp. 234.
5) Fabre JE, Nguyen M, Athirakul K, Coggins K, McNeish JD, Austin S, Parise LK, FitzGerald GA, Coffman TM, Koller BH. Journal of Clinical investigation, 2001, 107:603
6) Gross S,Tilly P, Hentsch D, Vonesch JL, Fabre JE. Journal of Experimental Medicine, 2007, 204:311
7) Medscape Early Penile Rehabilitation Helps Reduce Later Intractable ED.
8) LaBonde, MS, DVM, Jerry. "Avian Reproductive and Pediatric Disorders" (PDF). Michigan Veterinary Medical Association. Archived from the original on 2008-02-27.
9) Haché M, Denault A, et al. Inhaled epoprostenol (prostacyclin) and pulmonary hypertension before cardiac surgery. J Thorac Cardiovasc Surg 2003;125:642-649
10) Pharmacology 2007. Rang, Ritter, Dale, Flower. Churchill Livingstone Elsevier. Judiths Hopfer Deglin and April Hazard Vallerand (2006), Davis Drug Guide for Nurses,F.A Davis, Philadelphia, Pennsylvania, U.S.A Copyright, 1427 pages.
11) Sharma, I.; Dhaliwal, L.; Saha, S.; Sangwan, S.; Dhawan, V. (2010). "Role of 8-iso-prostaglandin F2alpha and 25-hydroxycholesterol in the pathophysiology of endometriosis". Fertility and Sterility 94 (1): 63–70.
12) Cheng Y et al., Role of prostacyclin in the cardiovascular response to thromboxane A2. “Science” 2002;296:539-51.
13) DR.S.R. Sree gouri. Posted date: 06-Sep-2011
14) Weksler, B.B.,et.al. 1977. Proc. Natl. sci. U.S.A. 74: 3922-3926
15) Health information from the Royal woman’s Hospital (Victoria Australia).
16) Chime tutorial: on action of COX enzyme with aspirin. Interactive Biochemistry was created by Edward K. O'Neil and Charles M. Grisham at the University of Virginia in Charlottesville,Virginia.
17) Principles of anatomy and physiology, Gerard Tortora, Sandra R. Grabowski. 2003, JohnWiley&sons.Inc.
18) D.F. Horrobin, The Lancet, volume 309, Issue 8018, pages 936-7, 30 April 1977.
19) David H Adler, John A Phillips, Joy D Cogan, Tina M Iverson, Nathalie Schnetz-Boutaud, Jeffrey A Stein, David A Brenner, Ginger L Milne, Jason D Morrow, Oliver Boutaud, John A Oates, Department of Medicine, Division of Clinical Pharmacology,
Vanderbilt University, Vanderbilt Medical Center, Journal of Gastroenterology (impact factor: 4.16). 01/2009: 44 suppl 19: 1-7.
20) Clark KE, Austin JE, Stys SJ. PubMed
21) Camras CB, Miranda OC Department of Ophthalmology, Mt. Sinai School of Medicine, New York, NY 10029. Progress in Clinical and Biological Research [1989, 312:197-210]
22) J.L. Gordon, D.E. MacINTYRE & R.M. McMillan, Department of pathology, University of Cambridge
23) DAVID W. STANLEY-SAMUELSON, Department ofEntomologica/Sciences, University of California, Berkeley, California 94720
24) Baird DT, Rodger M, Cameron IT, Roberts I. Source: Department of Obstetrics and Gynaecology, University of Edinburgh, U.K.









