About Author:
Ramandeep Kaur
Assistant Professor, CT Institute of Pharmaceutical Sciences,
Jallandhar- 143001
deepraman_18@yahoo.in
ABSTRACT
Phytosomes are little cell like structure which are also termed herbosomes which are formed by complexation of the polyphenolic phytoconstituents in molar ratio with phosphatidylcholine. Bioavailability of flavonoid containing phytomedicines, is reported to be low and erratic due to limited absorption, elevated presystemic metabolism and rapid elimination. But their phytosomal formulations show improved absorption, enhanced delivery and increased bioavailability of phytochemicals than the conventional herbal extracts containing dosage forms. Phytosome technology has been effectively used to enhance the bioavailability of many popular herbal extracts e.g. milk thistle, grape seed, green tea, hawthorn, etc. The improved pharmacokinetic and pharmacological properties of phytosomes make them suitable for the treatment of serious degenerative disorders. This article reviews the recent trends and applications of various herbal extract phytosomes as a tool of drug delivery system and as an emerging technology for increasing bioavailabity of phytoconstituents.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1399
INTRODUCTION
Herbal medicine is employed by mankind from the ancient times and during the last century chemical and pharmacological studies have been performed on a lot of plant extracts in order to know their chemical composition and to confirm their indications for treating diseases. There is a great interest and medical need for the improvement of bioavailability of a large number of herbal drugs and plant extracts which are poorly lipid soluble and so less bioavailable1. In human body, the phospholipids are also used as natural carriers for both fat-miscible and water miscible nutrients. Most of the plant constituents specifically phenolics are water soluble and the reason for less bioavailability is the inability to cross the lipid membranes of intestine. Their bioavailability can be improved with the use of different novel delivery systems like liposomes, marinosomes, niosomes and photosomes which can enhance the rate of release as well as the capacity to cross the lipid rich biomembranes2, 3. Recent technology - phytosome process, a breakthrough in herbal medicine, is being used to refine, enhance, and intensify the power of herbal medicines. Phytosome dietary supplements are the modern culmination of this great tradition. Phytosome is a patented technology developed by a leading manufacturer of drugs and nutraceuticals (Indena), which are prepared by incorporating standardized plant extracts or water soluble phytoconsituents into phospholipids to produce lipid compatible molecular complexes, in order to vastly improve their absorption and bioavailability4-7.
Phospholipids are small lipid molecules where glycerol is bonded to two fatty acids, while the third hydroxyl, normally one of the two primary methylenes, bears a phosphate group bound to a biogenic amino or to an amino acid. Phospholipids are a class of lipids and are a major component of all cell membrane. They are miscible both in water and in lipid environments, and are well absorbed orally. Phospholipids from soybean, (Glycine max) mainly phosphatidylcholine is a lipophilic agent that readily complex polyphenolics and widely employed to make phytosomes8.
Phosphatidylcholine, the major molecular building block of cell membranes is a compound miscible in both water and in oil/lipid environments9. The phytosomes has more ability to carry the herbal extract of hydrophilic nature through the lipid bilayer and thus its bioavailablity is more compared to simple extract. The phytosome technology has been reported to effectively enhance the bioavailability of many popular herbal extracts including milk thistle, Ginkgo biloba, grape seed, green tea, hawthorn, ginseng, turmeric, centella, ammi etc and can further be developed for various therapeutic uses or dietary supplements10, 11. The Phytosomes process itself produces a little cell whereby the valuable component of the herbal extract is protected from degradation by digestive secretions and gut bacteria12.
Common Drug Delivery Systems used in Pharmaceutical technology:
The “Somes” the cell like formulations of novel drug delivery system. There are different types of somes like
• Liposomes are nano size artificial vesicles of spherical shape that can be
produced from natural phospholipids and cholesterol.
• Herbosomes are purified phytochemical extracts complexed with phospholipids for a better bioavailability and enhanced biological activities.
• Cubosomes are nanoparticles but instead of the solid particles, cubosomes are self-assembled liquid crystalline particles of certain surfactant with proper ratio of water with a microstructure that provides unique properties of practical interest.
• Colloidosomes are solid microcapsules formed by the self assembly of colloidal particles at the interface of emulsion droplets which are hollow, elastic shells whose permeability and elasticity can be precisely controlled.
• Ethosomes are noninvasive delivery carriers that enable drugs to reach the deep skin layers and/or the systemic circulation. They contain phospholipids, alcohol (ethanol and isopropyl alcohol) in relatively high concentration and water.
• Aquasomes these are spherical 60300nm particles used for drug and antigen delivery. The particle core is composed of noncrystalline calcium phosphate or ceramic diamond, and is covered by a polyhydroxyl oligomeric film. Aquasomes were prepared by self-assembling of hydroxyapatite by co-precipitation method and thereafter preliminary coated with polyhydroxyl oligomers (cellobiose and trehalose) and subsequently adsorbed with bovine serum albumin (BSA) as a model antigen. BSA-immobilized aquasomes were around 200 nm in diameter and spherical in shape and had approximately 20-30% BSA-loading efficiency.
• Pharmacosomes are pure drug vesicles formed by the amphiphilic drugs. Any drug possessing a free carboxyl group or an active hydrogen atom (–OH, NH2) can be esterified (with or without a spacer group) to the hydroxyl group of a lipid molecule, thus generating an amphiphilic prodrug. The amphiphilic prodrug is converted to pharmacosomes on dilution with water.
• Niosomes are non-ionic surfactant vesicles and, as liposomes,are bilayered structures. Niosomes present low production cost, greater stability, andresultant ease of storage.Niosomes are chemically stable, can entrap both lipophilic and hydrophilic drugs either inaqueous layer or in vesicular membrane and present lowtoxicity because of their non-ionic nature.
• Proniosomes are dry formulations of surfactant-coated carrier, which can be measured out as needed and rehydrated by brief agitation in hot water.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
IMPORTANCE OF PHOSPHATIDYLCHOLINE IN PHYTOSOME TECHNOLOGY
Properties of Phosphatidylcholine:
* Phosphatidylcholine is a natural component of lecithin.
* Phosphatidylcholine is found throughout the human body as an essential component of cell membranes.
* The reaction of phosphatidylcholine with the herbal compounds creates new molecules known as phytosomes.
* Phosphatidylcholine is a bifunctional compound, the phosphatidyl moiety being lipophilic and the choline moiety being hydrophilic in nature. Specifically the choline head of the phosphatidylcholine molecule binds to these compounds while the lipid soluble phosphatidyl portion comprising the body and tail which then envelopes the choline bound material. Hence, the phytoconstituents produce a lipid compatible molecular complex with phospholipids, also called as phyto-phospholipid complex. Molecules are anchored through chemical bonds to the polar choline head of the phospholipids, as can be demonstrated by specific spectroscopic techniques13-14.
* The unit phytosome is usually a flavonoid molecule linked with at least one phosphatidylcholine molecule which results in a little micro sphere10.
* Phosphatidylcholine is a very interesting molecule. It contains a water-soluble head (choline component) with two long, fat soluble tails (phosphatidyl component). Because of this dual solubility, phosphatidylcholine is an extremely effective emulsifier. Emulsifiers are substances which can mix together two seemingly incompatible liquids, such as oil and water. The emulsifying action of phosphatidylcholine is often used to greatly increase the absorption of fat-soluble vitamins and drugs.
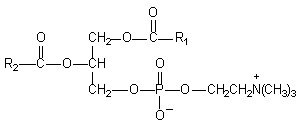
Figure1: Chemical Structure of Phosphatidylcholine
* Phosphatidylcholine functions in maintaining the "fluidity" of our cellular membranes.
* Phosphatidylcholine plays a critical role in all membrane dependent metabolic processes. For example, membrane-bound enzyme systems, such as those involved in energy production within specialized cell compartments known as mitochondria, depend on phosphatidylcholine for stimulation. If phosphatidylcholine levels are inadequate, these enzymes will not become active. Since the mitochondria produce energy for the entire cell, all cellular processes are adversely affected when this occurs.
* Phosphatidylcholine is a widely used pharmaceutical preparation in Europe for the treatment of liver disease and elevated cholesterol levels. In liver disease, phosphatidylcholine protects and enhances liver function; in high cholesterol, it improves the transport of cholesterol to the liver where it can be broken down.
* In the United States, phosphatidylcholine is regarded as a food supplement because no therapeutic claims are made by manufacturers.
METHODS OF PREPARATION
Different methods of preparation as repoted in literature are given below:
1. Yanyu et al. (2006) reported the preparation of silybin phytosome which involved the formation of silybin-phospholipid complex using ethanol as a reaction medium15. The required amounts of drug and phospholipids were placed in a 100 ml round?bottom flask and dissolved in anhydrous ethanol. After ethanol was evaporated off under vacuum at 40oC, the dried residues were gathered and placed in desiccators overnight, then crushed in the mortar and sieved with a 100 mesh. The resultant silybin– phospholipid complex was transferred into a glass bottle flushed with nitrogen and stored in the room temperature16.
2. Marena and Lampertico (1991), Jiang et al. (2001), Maiti et al. (2006) and Maiti et al. (2006) reported the methods of phytosome preparation17-20. According to literature procedures of phytosome preparations, phospholipids were selected from the group consisting of soy lecithin, from bovine or swine brain or dermis, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine in which acyl group was same or different and mostly derived from palmitic, stearic, oleic and linoleic acid. The flavonoids were selected from the group consisting of quercetin, kaempferol, quercretin-3, rhamnoglucoside, quercetin-3-rhamnoside, hyperoside, vitexine, diosmine, 3- rhamnoside, (+) catechin, (-) epicatechin, apigenin-7-glucoside, luteolin, luteolinglucoside, ginkgonetine, isoginkgonetine and bilobetine.
3. Another procedure for phytosome synthesis as given in literature involved the reaction of 3?2 moles but preferably, one mole of a natural or synthetic phospholipid, such as phosphatidylcholine, phosphatidylethanolamine or phosphatidyiserine with one mole of flavolignanans, either alone or in the natural mixture in aprotic solvent such as dioxane or acetone from which complex can be isolated by precipitation with non solvent such as aliphatic hydrocarbons or lyophilization or by spray drying. In the complex formation of phytosomes the ratio between these two moieties was in the range from 0.5?2.0 moles16. The most preferable ratio of phospholipid to flavonoids is 1:1
4. Naringenin–PC complex was prepared by taking naringenin with an equimolar concentration of phosphatidylcholine. The equimolar concentration of phosphatidylcholine and naringenin were placed in a 100 mL round bottom flask and refluxed in dichloromethane for 3 h. On concentrating the solution to 5– 10 mL, 30 mL of n?hexane was added to get the complex as a precipitate followed by filtration. The precipitate was collected and placed in vacuum desiccators21.
Common steps of preparation of phytosomes
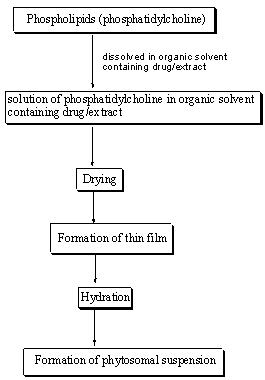
Figure 2: Common stages for preparation of phytosomes22
1. ADVANTAGES and disadvantages OF PHYTOSOMES23, 24, 25
Advantages
1. It enhances the absorption of lipid insoluble hydrophilic polar phytoconstituents through oral as well as topical route and increases the bioavailability.
2. It ensures appreciable drug entrapment.
3. As the absorption of active constituent(s) is improved, it reduces the dose requirement.
4. Phosphatidylcholine besides acting as a carrier, also acts as a hepatoprotective, hence it shows synergistic effect when hepatoprotective substances are employed.
5. Phytosomes have better stability profile because chemical bonds are formed between phosphatidylcholine molecule and phytoconstituent.
6. Application of phytoconstituents in form of phytosome improves their percutaneous absorption and act as functional cosmetics. Phytosome is widely used in cosmetics due to their more skin penetration and high lipid profile.
7. Phytosomes also have nutritional benefit of phospholipids.
8. The non?lipophilic phytoconstituent in phytosome can easily permeate the intestinal walls and is better absorbed in intestinal lumen.
9. By improving the solubility of bile to herbal constituent, liver targeting can be facilitated by phytosomes.
10. The phytosome process not only provides valuable phosphatidylcholine, it also intensifies the action of herbal compounds, by improving absorption, increasing biological activity, and enhancing delivery to the target tissue. Because of these effects, the phytosome is referred to as a delivery system.
Disadvantages
Phytoconstituent is rapidly eliminated from phytosomes. The duration of action is short.
PROPERTIES OF PHYTOSOMES
Chemical properties
Phytosomes is a complex between a natural product and natural phospholipids, like soy phospholipids which is obtained by reaction of stoichiometric amounts of phospholipid and the substrate in an appropriate solvent. It is confirmed by the spectroscopic analysis that the main phospholipid-substrate interaction is due to the formation of hydrogen bonds between the polar head of phospholipids (i.e. phosphate and ammonium groups) and the polar functionalities of the substrate. Phytosomes assumes a micellar shape forming liposomial-like structures in the presence of water.
Biological Properties
Phytosome, as an advanced form of herbal product is better absorbed, utilized and as a result produces better results than conventional herbal extract. The increased bioavailability of the phytosome over the non complexed botanical derivatives has been explained and verified by various pharmacokinetics and pharmacodynamic tests on experimental animal models and on human subjects26.
CHARACTERIZATION OF PHYTOSOMES26, 27
The Phytosomes are characterized by physical and biological tests and spectral analysis. The physical attributes include shape, size, its distribution, percentage drug capture, entrapped volume, percentage drug release, and chemical composition. Hence, the characterization of phytosomes is done by the following parameters:
1. Membrane permeability
2. Percent entrapped solutes
3. Chemical composition
4. Quantity and purity of the starting materials
I. Physical tests
1. Visualization: Visualization of phytosomes can be done using transmission electron microscopy (TEM) and by scanning electron microscopy (SEM) 28.
2. Particle size and zeta potential: The particle size and zeta potential can be determined by dynamic light scattering (DLS) using a computerized inspection system and photon correlation spectroscopy (PCS) 29.
3. Drug entrapment efficiency: The entrapment efficiency of a drug by phytosomes can be measured by the ultracentrifugation technique 30.
4. Transition temperature: The transition temperature of the phytosomes can be determined by differential scanning calorimetry 31.
5. Surface tension activity measurement: The surface tension activity of the drug in aqueous solution can be measured by the ring method in a Du Nouy ring tensiometer 32.
6. Vesicle stability: The stability of vesicles can be determined by assessing the size and structure of the vesicles over time. The mean size is measured by DLS and structural changes are monitored by TEM 33.
7. Drug content: The amount of drug can be quantified by a modified high performance liquid chromatographic method or by a suitable spectroscopic method 34.
II. Spectroscopic evaluations
To confirm the formation of a complex and interaction between the phytoconstituent and the phospholipids, the following spectroscopic methods are used 35.
1. 1HNMR
The NMR spectra of (+) ?catechin and its stoichio?metric complex with distearoyl phosphatidylcholine have been studied by Bombardelli et al 36. In nonpolar solvents, there is a marked change of the 1H?NMR signal originating from the atoms that is involved in the formation of the complex, without any summation of the signal peculiar to the individual molecules. The signals from the protons belonging to the flavonoids are to be broadened that the proton cannot be relieved. In phospholipids, there is broadening of all the signals while the singlet corresponding to the N?(CH3)3 of choline undergoes an upfield shift. By heating the sample to 60?, the 1H?NMR shows the appearance of some new broad bands, which correspond mainly to the resonance of the flavonoid moiety.
2. 13CNMR
In the 13C?NMR spectrum of (+) ?catechin and its stoichiometric complex with distearoyl phosphatidylcho?line, particularly when recorded in C6D6 at room temperature, all the flavonoid carbons are clearly invisible. The signals corresponding to the glycerol and choline portion of the lipid (between 60–80 ppm) are broadened and some are shifted, while most of the resonances of the fatty acid chains retain their original sharp line shape. After heating to 60?, all the very broad and partially overlapping signals belonging to the flavonoid moieties reappear.
3. FTIR
The formation of the complex can be also be analysed by IR spectroscopy by comparing the spectrum of the complex with the spectrum of the individual components and their mechanical mixtures. FTIR spectroscopy is also a useful tool for the control of the stability of phytosomes when micro?dispersed in water or when incorporated in very simple cosmetic gels.
III. Biological tests (in vitro and in vivo evaluations)
The selection of models for in?vitroand in?vivoevaluations is based on the expected therapeutic activity of biologically active phytoconstituents present in the phytosomes 35. For example, in vitro antihepatotoxic activity is done by evaluating the antioxidant and free radical scavenging activity of the phytosomes. For assessing antihepatotoxic activity in?vivo, the effect of prepared phytosomes on animals against thioacetamide?, paracetamol or alcohol?induced hepatoxicity can be examined37, 38. The in vivo safety evaluation of glycyrrhetinic acid?Phytosome® ointment, a commercial product, involves the skin sensitization and tolerability studies39.
DIFFERENCE BETWEEN PHYTOSOME AND LIPOSOME
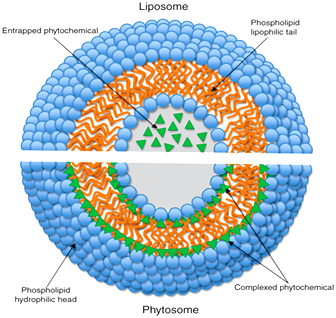
Fig. 3: Shows difference between liposome and phytosome.
The molecular organization of the liposome (upper segment)
The molecular organization of phytosomes (lower segment)
Liposomes are prepared by same procedure as phytosomes. A liposome is formed by mixing a water soluble substance with phosphatidylcholine in definite ratio under specific conditions. The main differences between liposomes and phytomes are:
1. Main difference is that in liposomes no chemical bond is formed and the phosphatidylcholine molecules surround the water soluble substance. In a liposome, the material is simply emulsified.
2. There may be hundreds or even thousands of phosphatidylcholine molecules surrounding the water-soluble compound. In contrast, with the phytosome process the phosphatidylcholine and the plant components actually form a 1:1 or a 2:1 molecular complex depending on the substance(s) complexes, involving chemical bonds. This difference results in better absorption of phytosomes than liposomes showing better bioavailability. Phytosomes have also been found superior to liposomes in topical and skin care products40 (Fig. 3).
3. Another difference is size of liposomes. Liposomes, although composed of phosphatidylcholine, are much larger.
4. Fundamental differences are that in liposomes, the active constituents are dissolved in the central part of the cavity, with no possibility of molecular interaction between the surrounding lipid and a hydrophilic substance41, 42. On the other hand the phytosome complex can somewhat be compared to an integral part of the lipid membrane, where the polar functionalities of the lipophilic molecule interact via hydrogen bonds with the polar head of a phospholipids (i.e. phosphate and ammonium groups), forming a unique pattern which can be characterized by spectroscopy43, 44, 45. This difference results in phytosome being much better absorbed than liposomes showing better bioavailability.
5. Phytosomes are also superior to liposomes in skin care products while the liposome is an aggregate of many phospholipids molecules that can enclose other phytoactive molecules but without specifically bonding to them.
6. Liposomes are touted delivery vehicles, but for dietary supplements, they are very less efficient. But for phytosome products numerous studies prove they are markedly better absorbed and have substantially greater clinical efficacy.
7. Some liposomal drug complexes operate in the presence of the water or buffer solution whereas phytosomes operate with the solvent having a reduced dielectric constant. Starting material of component like flavonoids is insoluble in chloroform, ethyl ether or benzene. They become extremely soluble in these solvents after forming phytosomes. This chemical and physical property change is due to the formation of a true stable complex46.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
APPLICATIONS OF PHYTOSOMES
Some of the safe and effectivemarketed phytosome products are given in Table 1.
Table 1: Commercial Phytosome Products47, 48, 49
|
Trade name |
Phytochemical |
Indication |
|
18ß-glycyrrhetinic acid Phytosome® |
18ß-glycyrrhetinic acid from licorice rhizome |
Soothing |
|
Boswellia Phytosome® |
Boswellic acids from Boswellia serrata's resins |
Anti-inflammatory |
|
Centella Phytosome® |
Triterpenes fromCentella asiatica leaf |
Cicatrizing, trophodermic |
|
Crataegus Phytosome® |
Vitexin-2″-O-rhamnoside from Hawthorn flower |
Antioxidant |
|
Escin ß-sitosterol Phytosome® |
Escin ß-sitosterol from horse chestnut fruit |
Anti-oedema |
|
Ginkgoselect® Phytosome® |
Ginkgoflavonglucosides, ginkgolides, bilobalide from Ginkgo biloba leaf |
Vasokinetic |
|
Ginselect® Phytosome® |
Ginsenosides from Panax ginseng rhizome |
Skin elasticity improver, adaptogenic |
|
Ginkgo biloba Terpenes Phytosome® |
Ginkgolides and bilobalide from Ginkgo biloba leaf |
Soothing |
|
Ginkgo biloba Dimeric Flavonoids Phytosome® |
Dimeric flavonoids from Ginkgo biloba leaf |
Lipolytic, vasokinetic |
|
Greenselect® Phytosome® |
Polyphenols from green tea leaf |
Prevention of free radical-mediated tissue damages and weight management |
|
Leucoselect® Phytosome® |
Polyphenols from grape seed |
Antioxidant, capillarotropic |
|
Meriva® |
Curcuminoids from turmeric rhizome |
Anti-inflammatory |
|
PA2 Phytosome® |
Proanthocyanidin A2 from horse chestnut bark |
Anti-wrinkles, UV protectant |
|
Resveratrol Phytosome® |
Resveratrol from Polygonum cuspidatum's rizhome |
Antioxidant |
|
Sericoside Phytosome® |
Sericoside from Terminalia sericea bark root |
Anti-wrinkles |
|
Siliphos® |
Silybin from milk thistle seed |
Hepatocyte protection |
|
Silymarin Phytosome® |
Silymarin from milk thistle seed |
Antihepatotoxic |
|
Virtiva® |
Ginkgoflavonglucosides, ginkgolides, bilobalide from Ginkgo biloba leaf |
Vasokinetic |
|
Visnadex® |
Visnadin from Amni visnaga umbel |
Vasokinetic |
|
LeucoselectTM Phytosome |
Procyanidolic oligomers from grape seeds |
Systemic antioxidant, diabetes, varicose veins, protection against heart disease |
|
SilybinTM Phytosome |
Silybin from milk thistle (Silymarin) |
Liver disease |
|
HawthornTM Phytosome |
Flavonoids |
heart disease |
|
GlycyrrhizaTM Phytosome |
18-ß-glycyrrhetinic acid |
Anti-inflammatory action |
|
MirtoselectTM Phytosome |
Anthocyanoside from an extract of Bilberry |
Improves capillary tone, reduce abnormal blood vessel permeability and is potent antioxidant. They hold great potential for the management of retinal blood vessel problems and venous insufficiency. |
|
SabalselectTM Phytosome |
An extract of saw palmetto berries through supercritical CO2 extracts |
It delivers fattyacids, alcohols and sterols that benefit prostate, heart and is used in non cancerous prostate enlargement. |
|
PolinaceaTM Phytosome |
Echinacosides and unique HMW polysaccharides from Echinacea angustifolia |
It enhances immune function in response to toxic challenge |
|
OleaselectTM Phytosome |
Polyphenols from olive oil |
As potent antioxidant, inhibit harmful oxidation of LDL cholesterol and also have anti-inflammatory activity. |
|
LymphaselectTM Phytosome |
A standard extract of Melilotus officinalis |
Indicated for venous disorders, including chronic venous insufficiency of lower limbs |
Some patented technologies related to phytosomes
There is lot of research work done on phytosomes by number of academic scientists as well as by industrial laboratories due their wide list of applications. Some patents for phytosomes and other related technologies along with their applications and innovations are listed in Table 2.
Table 2: Some patented technologies related to phytosomes
|
Title of patent |
Innovation |
Patent No |
Reference |
|
Phospholipid complexes of olive fruits or leaves extracts having improved bioavailability |
Phospholipids complexes of olive fruits or leaves extracts or compositions containing it having improved bioavailability |
EP/1844785 |
50 |
|
Compositions comprising Ginkgo biloba derivatives for the treatment of asthmatic and allergic conditions |
Compositions containing fractions deriving from Ginkgo biloba, useful for the treatment of asthmatic and allergic conditions |
EP1813280 |
51 |
|
Fatty acid monoesters of sorbityl furfural and compositions for cosmetic and dermatological use |
Fatty acid monoesters of sorbityl furfural selected from two diff series of compounds in which side chain is a linear or branched C3 -C19 alkyl radical optionally containing at least one ethylenic unsaturation |
EP1690862 |
52 |
|
Cosmetic and dermatological composition for the treatment of aging or photodamaged skin |
Composition for topical treatment of the skin comprises a substance that stimulates collagen synthesis and a substance that enhances the interaction between extracellular matrix and fibroblasts Cosmetic or dermatological composition for topical treatment |
EP1640041 |
53 |
|
Treatment of skin, and wound repair, with thymosin beta 4 |
Compositions and methods for treatment of skin utilizing thymosin β4. |
US/2007/ 0015698 |
54 |
|
Soluble isoflavone compositions |
Isoflavone compositions exhibiting improved solubility (e.g., light transmittance), taste, color, and texture characteristics, and methods for making the same |
WO/2004/ 045541 |
55 |
|
An anti-oxidant preparation based on plant extracts for the treatment of circulation and adiposity problems |
Preparation based on plant extracts which has an anti-oxidant effect and is particularly useful in treatment of circulation problems such as phlebitis, varicose veins, arteriosclerosis, haemorrhoids and high blood pressure |
EP1214084 |
56 |
|
Complexes of saponins with phospholipids and pharmaceutical and cosmetic compositions containing them |
Complexes of saponins with natural or synthetic phospholipids have high lipophilia and improved bioavailability and are suitable for use as active principle in pharmaceutical, dermatologic and cosmetic compositions |
EP0283713 |
57 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
Phytomedicine or herbal medicine is the science, art, and exploration of using botanical remedies to treat illness. Treatment of disease with plants has been popular since ancient times. Phytosomes are advanced forms of herbal products that are better absorbed, utilized, and as a result produce better results than conventional herbal extract. Phytosomes are formed by complexing phytoconstituents and phospholipids in 1:1 and 2:1 ratio. Phytosomes have proved itself as a better drug delivery system than liposomes. The Phytosome process has been applied to many popular herbal extracts including Ginkgo biloba, grape seed, hawthorn, milk thistle, green tea, and ginseng. This review concludes that phytosome show variety of applications e.g. they are used to treat the pain and symptoms associated with asthma, arthritis, rheumatism, ulcers, phlebitis, edema, varicose veins, premenstrual syndrome, diabetic retinopathy and hemorrhoids. Phytosomes are used as a medicament and have wide scope in cosmetic technology.
REFERENCES
1. Manach C, Scalbert A and Morand C. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004; 79:727-47.
2. Uchegbu IF and Vyas SP. Non-ionic surfactant based vesicles. (niosomes) in drug delivery. Int J Pharmaceu 1998; 172:33–70.
3. Moussaoui N, Cansell M and Denizot A. Marino-somes: marine lipid-based liposomes: physical characterization and potential applications in cosmetics.Int J Pharmaceu 2002; 242:361-365.
4. Bombardelli E, Curri SB, LoggiaDella R, Del NP, Tubaro A and Gariboldi P. Complexes between phospholipids and vegetal derivatives of biological interest. Fitoterapia 1989; 60:1-9.
5. Mauri PL, Simonetti P, Gardana C, MinoggioM,Morazzoni P and Bombardelli E. Liquid chromatography/atmospheric pressure chemical ionization mass spectrometry of terpene lactones in plasma of volunteers dosed with Ginkgo biloba L. extracts. Rapid Commun Mass Spectrom 2001; 15:929–34.
6. Kidd PM and Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin–phosphatidylcholine complex (Siliphos®). AlterMed Rev 2005; 10: 193–203.
7. Rossi R, Basilico F, Rossoni G, Riva A, Morazzoni P and Mauri PL: Liquid Chromatography/atmospheric pressure chemical ionization ion trap mass spectrometry of bilobalide in plasma and brain of rats after oral administration of its phospholipidic complex. J Pharm Biomed Anal 2009; 50: 224–7.
8. Citernesi U. and Sciacchitano M: Phospholipids/active ingredient complexes. Cosm & Toil 1995; 110(11):57-68.
9. Kidd PM. Phosphatidylcholine: A superior protectant against liver disease. Altern Med Rev 1996;1:258?74.
10. Murray, Available at: doctormurray.com/articles/silybin.htm Accessed- Sept. 28, 2008.
11. Vitamedics, Phytosome Products, Available at vitamedics.com. Accessed Sept. 19, 2008.
12. Murray; Phytosomes-Increase the absorption of herbal extract. Available at www.doctormurray. com/articles/Silybinhtm.Accessed-Januar18, 2006.
13. Bombardelli E. Phytosome: New Cosmetic Delivery System. Boll Chim Farm. 1991; 130:431-38.
14. Bombardelli E, Spelta M. Phospholipid-Polyphenol Complexes: A New Concept in Skin Care Ingredients. Cosm & Toil.1991; 106:69- 76.
15. Yanyu X, Yunmei S, Zhipeng C, Qineng P. The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats, International Journal of Pharmaceutics, volume307, (2006) page no77–82.
16. Maghraby GMM, Williams AC, Barry BW. Oestradiol skin delivery from ltradeformable liposomes: refinement of surfactant concentration. Int. J. Pharm., 2000, 196: 63?74.
17. Jiang YN, Yu ZP, Yan ZM and Chen JM: Studies on preparation of herba epimedii flavanoid phytosomes and their pharmaceutics. Zhongguo Zhong Yao Za Zhi 2001; 26(2): 105-8.
18. Maiti K, Mukherjee K, Gantait A, Saha BP and Mukherjee PK: Curcumin– phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm 2007; 330:155–63.
19. Maiti K, Mukherjee K, Gantait A, Saha BP and Mukherjee PK: Enhanced therapeutic potential of naringenin–phospholipid complex in rats. J Pharm Pharmacol. 2006;58: 1227– 33.
20. Sharma S. and Sikarwar M: Phytosome: a review, Planta Indica. 20051(2), 1-3.
21. Jain. N. K. Liposomes as drug carriers, controlled and novel drug delivery, 1st edition, CBS publisher, 2005, 308.
22. Fry, D. W. White J. C., Goldman I. D. Rapid secretion of low molecular weight solutes from liposomes without dilution. Anal. Biochem., 1978, 90: 809?815.
23. Dayan N, Touitou E. Carrier for skin delivery of trihexyphenidyl HCl: ethosomes vs liposomes. Biomaterials, 2002, 21:1879?1885.
24. Facino R. M., Carini M., Aldini G., et al. Free radicals sea action and anti?enzyme activities of procyanidines vitis vinifera?a mechanism for their capillary protection. Arzneim. Forsch., 1994, 44: 592?601.
25. Semalty A, Semalty M, Singh R, Rawat MSM. Phytosomes in herbal drug delivery. Indian drugs, 2006, 43: 937?946.
26. Bombardelli E, Mustich G. Bilobalide?phospholipid comlex, their uses and formulation containing them. U. S. Patent No. EPO?275005, 1991.
27. Abrol S., Trehan A., Katare O. P. Comparative study of different silymarin formulations: formulation, characterization and in vitro/in vivo evaluation. Current Drug Delivery, 2005, 2: 45?51.
28. Comoglio A., Tomasi A., Malandrino S., et al. Scavenging effect of silipide?A new silybinphospholipid complex, on ethanolderived free radicals. Biochem. Pharmacol., 1995, 50: 1313? 1316.
29. Delgi U., Urbino S. D. Tolerability and cutaneous sensitization study in healthy volunteers after topical application of the product glycyrrhetinic acid?Phytosome® ointment. Unpublished data submitted by CTFA, 2004, 36: 2.
30. Pandey S, Patel K. Phytosomes: Technical revolution in phytomedicine. Int J Pharma Tech Res 2010;2:627?31.
31. Jain N et. al. Phytosome: A Novel Drug Delivery System for Herbal Medicine. International Journal of Pharmaceutical Sciences and Drug Research 2010; 2(4): 224-228.
32. Battacharya S. Phytosomes: Emerging strategy in delivery of herbal drugs and nutrceuticals. Pharm Times 2009; 41:3.
33. Chauhan NS, Gowtham R, Gopalkrishna B. Phytosomes: A potential Phyto?phospholipid carriers for herbal drug delivery. J Pharm Res 2009;2:1267?70.
34. Bhattachrya S. Phytosomes: The new technology for enhancement of bioavailability of botanicals and nutraceuticals. Int J Health Res 2009;2:225.
35. Hikino H, Kiso Y, Wagner H, Fiebig M. Antihepatotoxic actions of flavonolignans from Silybum marinum fruits. Planta Med 1984;50:248?50.
36. Valenzula A, Aspillaga M, Vial S, Guerra R. Selectivity of Silymarin on the increase of the glutathione content on different tissues of the rat. Planta Med 1989;55:420?2.
37. Wellington K, Jarvis B. Silymarin: A review of its clinical properties in the management of hepatic disorders. Bio Drugs 2001;15:465?89.
38. Changediya V. et. al. Phytosomes: New Approach for Delivering Herbal Drug with Improved Bioavailability. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2011; Volume 2 Issue 3 Page No. 57.
39. Tedeco D, Steidler S, Galletti S, Tameni M, Sonzogni O, Ravarotto L. Efficacy of Silymarin ? Phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poult Sci 2004;83:1839?43.
40. Phytosomes: A Technical Revolution in Phytomedicine [online]. 2010 [cited 2010 Mar 22]. Available from: URL: http:// www.indena.com
41. Bombardelli E. Phytosomes in functional cosmetics. Fitoterapia 1994; 5: 387-401.
42. Bombardelli E, Curri SB, Gariboldi P and Gariboldi: Cosmetic utilization of complexes of Panax ginseng saponins with phospholipids in PHYTOSOME®form. Fitoterapia 1989;60:55–70 [Suppl. to issue N.1].
43. Bombardelli E, Curri SB, Della Loggia R, Del Negro P, Tubaro A and Gariboldi P: Anti-inflammatory activity of 18-â-glycyrrhetinic acid in PHYTOSOME® form. Fitoterapia 1989;60:29–37 [Suppl. to issue N.1].
44. Bombardelli E, Della LR ,Del NP, Tubaro A, Gariboldi P and Piergentili A: Topical antiinflammatory activity of complexes of escin and sterols with phospholipids. Part Fitoterapia 1989; 60:39–44 [Suppl. to issue N.1].
45. Gabetta B, Zini GF and Pifferi G: Spectroscopic studies on IdB 1016, a new flavolignan complex. Planta Med 1989; 55:615.
46. Barani H and Montazer M. A Review on Applications of Liposomes in Textile Processing. J Lip Res 2008; 18: 249-262.
47. Murray M T. Phytosomes: herbal support- increases the absorption of herbal extract, available at doctormurray.com/articles/silybin.html, 2004.
48. Kidd P M, Phytosome products. Available at indena.com
49. Joshi A, Chaturvedi S, Kumar V et. al. Phytosomes- A revolution in herbal drugs. Pharma Review. Kongposh publications. Dec. 2007- Jan. 2008.
50. Franceschi F, Giori A. A phospholipid complex of olive fruits or leaves extracts having improved bioavailability. EP1844785, 2007.
51. Di Pierro F. Compositions comprising Gingko biloba derivatives for the treatment of asthmatic and allergic conditions. EP1813280, 2007.
52. Bertelli V. Fatty acid monoesters of sorbityl furfural and compositions for cosmetic and dermatological use. EP1690862, 2006.
53. Doering T, Traeger A, Waldmann-Laue M. et al. Cosmetic and dermatological composition for the treatment of aging or photodamaged skin. EP1640041, 2006.
54. Kleinman H K, Goldstein A L, et al. Treatment of skin, and wound repair, with thymosin beta 4. U. S. Patent No-20070015698, 2007.
55. Khare A B. Soluble isoflavone compositions. WO/2004/ 045541, 2004.
56. Merizzi G. An anti-oxidant preparation based on plant extracts for the treatment of circulation and adiposity problems. EP1214084, 2002.
57. Bombardelli E, Patri G F, Pozzi R. Complexes of saponins with phospholipids and pharmaceutical and cosmetic compositions containing them. EP0283713, 1988.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









