 About Authors: Mayank Panchal1*, Biren Shah1, Krishna Murti1, Megha Shah1
About Authors: Mayank Panchal1*, Biren Shah1, Krishna Murti1, Megha Shah1
1*Department of Pharmacognosy,
Vidyabharti Trust College of Pharmacy,
Umrakh, (Gujarat) INDIA
Reference ID: PHARMATUTOR-ART-1081
Abstract
Moringa oliefera Lam. is one of the best known and most widely distributed and naturalized species of a monogenetic family Moringaceae. The roots of plant were extracted with ethanol by soxhlet technique and aqueous extract of roots are prepared by maceration technique. Both the extracts are subjected to phytochemical screening. Require quantity of alcoholic and aqueous extracts were obtained from the plant which is used as test drug in Streptozotocin induced diabetic model. The present investigation is undertaken to study the effect of the potential hypoglycemic effect of Moringa oliefera Lam. in Streptozotocin (STZ) induced Diabetes Mellitus.
[adsense:336x280:8701650588]
Introdution
Drumstick tree, also known as horseradish tree and ben tree in English, is a small to medium-sized, evergreen or deciduous tree native to northern India, Pakistan and Nepal. It is cultivated and has become naturalized well beyond its native range, including throughout South Asia, and in many countries of Southeast Asia, the Arabian Peninsula, tropical Africa, Central America, the Caribbean and tropical South America. The tree usually grows to 10 or 12 m in height, with a spreading, open crown of drooping, brittle branches, feathery foliage of tripinnate leaves, and thick, corky, deeply fissured whitish bark. It is valued mainly for its edible fruits, leaves, flowers, roots, and seed oil, and is used extensively in traditional medicine throughout its native and introduced ranges. Drumstick tree is indigenous to the Himalayan foothills of South Asia from northeastern Pakistan to northern West Bengal State in India and northeastern Bangladesh where it is commonly found from sea level to 1,400 m on recent alluvial land or near riverbeds and streams.
Moringa oliefera is a small, fast-growing evergreen or deciduous tree that usually grows up to 10 or 12 m in height. It has a spreading, open crown of drooping, fragile branches, feathery foliage of tripinnate leaves, and thick, corky, whitish bark. This rapidly-growing tree (also known as the horseradish tree, drumstick tree, benzolive tree, kelor, marango, mlonge, moonga, mulangay, nebeday, saijhan, sajna or Ben oil tree), was utilized by the ancient Romans, Greeks and Egyptians; it is now widely cultivated and has become naturalized in many locations in the tropics. It is a perennial softwood tree with timber of low quality, but which for centuries has been advocated for traditional medicinal and industrial uses. It is already an important crop in India, Ethiopia, the Philippines and the Sudan, and is being grown in West, East and South Africa, tropical Asia, Latin America, the Caribbean, Florida and the Pacific Islands. All parts of the Moringa tree are edible and have long been consumed by humans. The root is laxative, expectorant, diuretic, and good for inflammations, throat, bronchitis, piles, cures stomatitis, urinary discharges and obstinate asthma1.
According to Fuglie, the many uses for Moringa include: alley cropping (biomass production), animal forage (leaves and treated seed-cake), biogas (from leaves), domestic cleaning agent (crushed leaves), blue dye (wood), fencing (living trees), fertilizer (seedcake), foliar nutrient (juice expressed from the leaves), green manure (from leaves), gum (from tree trunks), honey- and sugar cane juice-clarifier (powdered seeds), honey (flower nectar), medicine (all plant parts), ornamental plantings, biopesticide (soil incorporation of leaves to prevent seedling damping off), pulp (wood), rope (bark), tannin for tanning hides (bark and gum), water purification (powdered seeds). Moringa seed oil (yield 30- 40% by weight), also known as Ben oil, is a sweet non-sticking, non-drying oil that resists rancidity. It has been used in salads, for fine machine lubrication, and in the manufacture of perfume and hair care products. In the West, one of the best known uses for Moringa is the use of powdered seeds to flocculate contaminants and purify drinking water, but the seeds are also eaten green, roasted, powdered and steeped for tea or used in curries. This tree has in recent times been advocated as an outstanding indigenous source of highly digestible protein, Ca, Fe, Vitamin C, and carotenoids suitable for utilization in many of the so-called “developing” regions of the world where undernourishment is a major concern2.
Moringa oliefera Lam contains several phytochemicals, some of which are of high interest because of their medicinal value, in particular this plant family is rich in a fairly unique group of glycoside compounds called glucosinolates and isothiocyanates3. The root, best known in India and the Far East, is extremely pungent. When the plant is only 60 cm tall, it can be pulled up, its root scraped, ground up and vinegar and salt added to make a popular condiment much like true horseradish. The root bark must be completely removed since it contains two alkaloids allied to ephedrine - benzylamine (moringine), which is not physiologically active, and the toxic moringinine which acts on the sympathetic nerve endings as well as on the cardiac and smooth muscles all over the body. Also present is the potent antibiotic and fungicide, pterygospermin. The alkaloid, spirachin (a nerve paralyzant) has been found in the roots. Even when free of bark, the condiment, in excess, may be harmful. (The key words are "in excess"- the body can detoxify small amounts of a great many things)2.
Recent studies demonstrate that isothiocyanates have antitumor activity in cancers of the lung, breast, skin, esophagus, and pancreas4,5. Small proteins/ peptides were isolated from the leaves of Moringa oliefera possessing antifungal and antibacterial activity6. The methanolic extract of the root (ME) contains moringine and moringinine which are reported
to possess analgesic and anticonvulsive activity7.
A crude methanol extract of the root was also screened for anti- inflammatory effect using the rat paw edema8. Aqueous, methanol (80%) and ethanol (70%) extracts of freeze-dried leaves showed radical scavenging and antioxidant activities9. The methanolic extract of the root exhibited significant CNS depressant activity in Mice10. Aqueous and alcoholic extracts of root and flower of this plant were screened for antihepatotoxic activity in paracetamol treated albino rats11.
So, in the traditional system of medicine, the plant is used for various health problems and diseases12. However, antidiabetic studies of the fresh roots of M. oliefera Lam. have been conducted so far to substantiate this practice.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
MATERIALS AND METHODS
Materials
Aerial parts of Moringa oliefera Lam. growing in natural habitant in Modasa, Gujarat, India, was collected in January, 2010 and identified by Dr. H. B. Singh, Taxonomist, NISCAIR, New Delhi. Voucher specimen of the plant (NISCAIR 1650/248) has been submitted in the institute for future reference purpose.
Preparation of the root extract
Dried and coarsely 500 g powdered roots of Moringa oliefera was extracted with 90% (v/v) ethanol in soxhlet apparatus for 36 hrs and aqueous extract was prepared by using maceration technique of extraction. Filter the filtrate. The filtrate was concentrated on water bath using petridish. The temperature was maintained at 55 0C. Dry and weigh the powdered extract.
The preliminary phytochemical analysis
The preliminary phytochemical studies were performed for testing different chemical groups present in ethanolic extract13-16.
Animals
Wistar albino rats of either sex weighing between 180 and 200 g were obtained from Jay Research Foundation, Vapi. The study was approved by the Institutional Ethics Committee for animal experimentation VBTCP (VBTCP/IEAC/10/07/20), Umrakh and all the procedures on animals were carried out as per CPCSEA guidelines, India. These animals were used for the antidiabetic activity studies. The animals were stabilized for 1 week. They were maintained in standard conditions at room temperature, 60±5% relative humidity and 12 h light dark cycle. They had been given standard pellet diet and water ad libitum throughout the course of the study.
Induction of Diabetes
Diabetes in rats was induced by intraperitoneal injection of Streptozotocin (60 mg/kg body weight) prepared in 0.1M citrate buffer (pH 4.5). Diabetes was confirmed by the determination of fasting blood glucose level on the second day after 48 hrs of administration of Streptozotocin. The animals with blood glucose levels from 180 to 200 mg/dl was be segregated and kept into cages marked with groups 2 to 5. The body weights of all the rats were determined on the first and 21th days of the experiment. The drug preparations were fed orally by oral feeding needle to rats of respective groups (groups 3-5) once daily for 21 days17.
Experimental procedure
Albinos Wistar rats initially weighing more than 150 gm were used for the experiments. Prior to dietary manipulation, all rats were fed standard pellet rodent diet and water ad libitum. After acclimatization, the rats were divided into five groups of six animals each.
Total duration of study was 21 days and the animals used were rendered diabetic by injecting Streptozotocin through intra-peritoneal (i.p.) at the dose of 60 mg/kg body weight Animals having blood glucose level more than 300 mg/dl were separated. During the period of study and at the end of the treatment period, blood samples were collected by retro-orbital vein of rat, after 16 hours fasting, for biological estimations on 3rd, 4th, 7th and 21th day of experiment; then rats were sacrificed by excess ether anesthesia and histopathology of kidney and liver was carried out.
The above treatments were given for a period of 21 days both in diabetic and non-diabetic animals.
Group 1 Normal control group
Group 2 Diabetic control group
Group 3 Diabetic rats treated with aqueous extract of Moringa oliefera
Group 4 Diabetic rats treated with ethanolic extract of Moringa oliefera
Group 5 Diabetic rats treated with Glipizide18 (2.5mg/kg)
Procedure for blood Glucose Monitoring
Blood was taken from the retro-orbital vein. Blood glucose estimation was carried out in laboratory. Effects of various extracts on blood glucose level in diabetic and non-diabetic rats have been shown in Tables.
Body weight measurement:
Body weight was measured totally ten times during the course of study period (i.e., on before Streptozotocin induction), using a Digital weighing scale. Effects of various extracts on body weight have been shown in Tables.
Statistical Analysis
Results obtained from model was expressed as mean±SEM and compared with the corresponding control values. P- Values were calculated by one way ANOVA by comparing with control. The final hypoglycemic activity was calculated by applying PRISM 5.0 Software computer package.
RESULTS
Phytochemical Analysis
On preliminary phytochemical screening the extract showed that the roots of M. oliefera Lam. contain alkaloids, flavanoids and tannins, while other constituents like carbohydrates, amino acids, glycosides and saponins were showed absent. Results are shown in table 1.
Table 1: Physical parameter of M. oliefera Lam. roots
|
Sr. No. |
Physical Parameters |
Results in Percentage* |
|
1 |
Total ash |
7.5 ± 0.003 |
|
2 |
Acid-insoluble ash |
3.6 ± 0.002 |
|
3 |
Water soluble ash |
2.5 ± 0.001 |
|
4 |
Sulfated ash |
1.1 ± 0.001 |
|
5 |
Alcohol soluble extractive |
17.6 ± 0.008 |
|
6 |
Water soluble extractive |
16.8 ± 0.007 |
|
7 |
PetroleumEther soluble extractive |
3.2 ± 0.002 |
|
8 |
Chloroform soluble extractive |
4.5 ± 0.003 |
|
9 |
Loss on drying |
0.91 ± 0.001 |
|
10 |
pH |
7.21 ± 0.004 |
*= Average of three reading
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
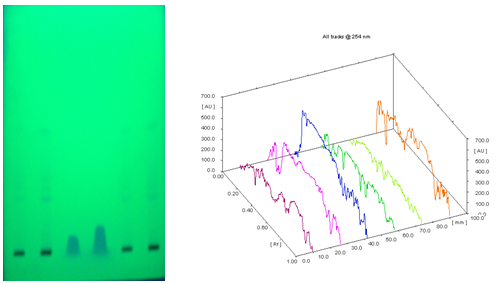
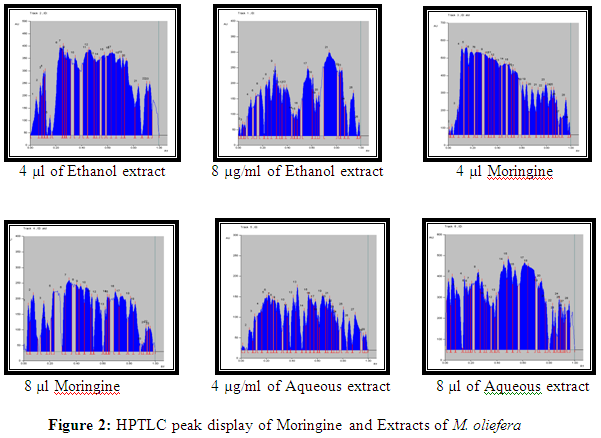
Table 2: Chromatogram of Moringine, ethanol and aqueous extract of M. oliefera Lam. roots.
|
Peak |
Track 1 |
Track 2 |
Track 3 |
Track 4 |
Track 5 |
Track 6 |
||||||
|
|
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
|
1 |
0.12 |
852.0 |
0.14 |
783.9 |
0.15 |
735.0 |
0.12 |
642.0 |
0.12 |
801.4 |
0.16 |
1801.4 |
|
2 |
0.18 |
730.2 |
0.18 |
836.0 |
0.26 |
892.0 |
0.17 |
3555.0 |
0.18 |
6658.3 |
0.22 |
1217.1 |
|
3 |
0.22 |
1373.1 |
0.23 |
701.5 |
0.33 |
4125.1 |
0.26 |
2323.6 |
0.23 |
8509.4 |
0.27 |
3954.1 |
|
4 |
0.33 |
2331.2 |
0.29 |
4380.6 |
0.42 |
7528.3 |
0.33 |
2304.9 |
0.33 |
7006.5 |
0.33 |
2116.7 |
|
5 |
0.37 |
2077.7 |
0.33 |
1955.1 |
0.51 |
9544.9 |
0.41 |
5312.0 |
0.42 |
6600.9 |
0.38 |
2407.0 |
|
6 |
0.46 |
2637.9 |
0.40 |
6216.1 |
0.60 |
5725.7 |
0.47 |
3811.5 |
0.52 |
5914.1 |
0.45 |
2348.9 |
|
7 |
0.54 |
891.1 |
0.52 |
4480.1 |
0.69 |
3795.3 |
0.54 |
3572.1 |
0.62 |
10705.1 |
0.57 |
5894.6 |
|
8 |
0.62 |
5689.6 |
0.61 |
2458.0 |
0.77 |
3427.4 |
0.61 |
9718.2 |
0.69 |
3009.3 |
0.61 |
946.3 |
|
9 |
0.77 |
2361.5 |
0.71 |
1812.2 |
0.82 |
7945.1 |
0.70 |
6468.8 |
0.76 |
4912.2 |
0.71 |
548.6 |
|
10 |
0.87 |
6370.2 |
0.82 |
1180.8 |
0.91 |
3179.7 |
0.79 |
6028.2 |
0.83 |
3423.7 |
0.79 |
2491.1 |
|
11 |
0.86 |
10138.4 |
0.87 |
2434.1 |
0.84 |
5640.1 |
0.95 |
2774.0 |
0.87 |
3118.8 |
||
|
12 |
|
|
0.92 |
6010.5 |
|
|
0.91 |
2282.6 |
|
|
0.95 |
1519.0 |
Result showed that the Rf value of standard Moringine was 0.33 and 0.60. Ethanol and aqueous extract of M. oliefera Lam. roots also showed a substance at Rf. 0.33 and around 0.61 respectively. So it was suggested that the compound separated at Rf 0.33 in ethanol extract and 0.60 in aqueous extract of M. oliefera Lam. roots may Moringine. Results are shown in table 2.
HPTLC photograph, chromatogram 3-D image of ethanol extract, Moringine and aqueous extract of M. oliefera Lam. roots are given in Figure 1 and 2.
[Track 1: 4 µl of Ethanol extract; Track 2: 8 µl of Ethanol extract; Track 3: 4 µl Moringine; Track 4: 8 µl Moringine; Track 5: 4 µl of Aqueous extract; Track 6: 8 µl of Aqueous extract]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
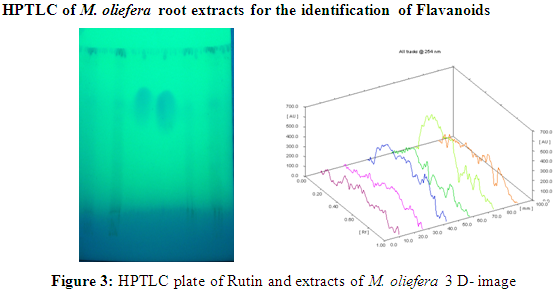
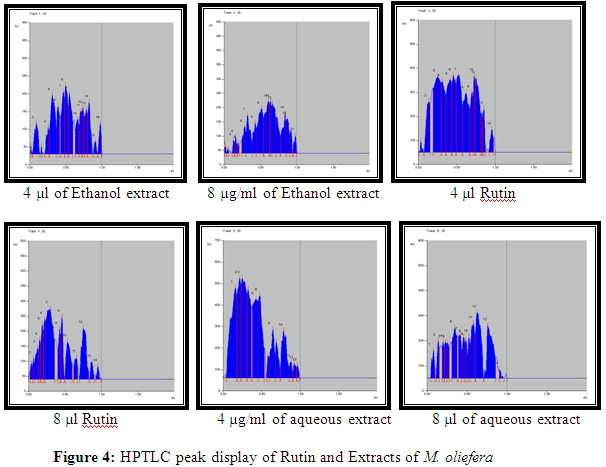
Table 3: Chromatogram of Rutin, ethanol and water extract of M. oliefera Lam. roots.
|
Peak |
Track 1 |
Track 2 |
Track 3 |
Track 4 |
Track 5 |
Track 6 |
||||||
|
|
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
Rf |
AUC |
|
1 |
0.12 |
752.0 |
0.11 |
744.9 |
0.14 |
843.0 |
0.13 |
1642.0 |
0.14 |
991.4 |
0.16 |
901.4 |
|
2 |
0.17 |
930.2 |
0.18 |
701.0 |
0.26 |
3455.0 |
0.14 |
2555.0 |
0.15 |
698.3 |
0.24 |
4817.1 |
|
3 |
0.21 |
1573.1 |
0.23 |
934.5 |
0.34 |
1425.1 |
0.26 |
7343.6 |
0.26 |
5809.4 |
0.25 |
3954.1 |
|
4 |
0.30 |
2631.2 |
0.29 |
3480.6 |
0.42 |
5728.3 |
0.36 |
2304.9 |
0.35 |
8006.5 |
0.34 |
2316.7 |
|
5 |
0.35 |
2077.7 |
0.35 |
9155.1 |
0.55 |
10544.9 |
0.41 |
3512.0 |
0.44 |
9600.9 |
0.37 |
2427.0 |
|
6 |
0.42 |
2837.9 |
0.40 |
6216.1 |
0.64 |
7525.7 |
0.45 |
8411.5 |
0.55 |
6414.1 |
0.47 |
2348.9 |
|
7 |
0.55 |
991.1 |
0.52 |
4780.1 |
0.63 |
7395.3 |
0.54 |
6572.1 |
0.66 |
1005.1 |
0.59 |
4894.6 |
|
8 |
0.62 |
4689.6 |
0.61 |
2858.0 |
0.72 |
4327.4 |
0.64 |
8318.2 |
0.70 |
2909.3 |
0.60 |
786.3 |
|
9 |
0.70 |
3161.5 |
0.71 |
8312.2 |
0.81 |
9745.1 |
0.71 |
1478.8 |
0.75 |
4512.2 |
0.72 |
10848.6 |
|
10 |
0.89 |
8370.2 |
0.82 |
2480.8 |
0.96 |
10179.7 |
0.73 |
9028.2 |
0.84 |
4323.7 |
0.79 |
3291.1 |
|
11 |
0.91 |
1138.4 |
0.87 |
1134.1 |
0.84 |
7640.1 |
0.91 |
7274.0 |
0.84 |
4318.8 |
||
|
12 |
|
|
0.92 |
6710.5 |
|
|
0.91 |
3282.6 |
|
|
0.95 |
1019.0 |
Result showed that the Rf value of standard rutin was 0.35 and 0.70. Ethanol and aqueous extract of M. oliefera Lam. roots also showed a substance at Rf. around 0.35 and 0.71 respectively. So it was suggested that the compound separated at Rf 0.35 in ethanol extract and at Rf 0.70 in aqueous extract of M. oliefera Lam. roots may Rutin. Results are shown in table 3.
HPTLC photograph, chromatogram 3-D image of ethanol extract, Rutin and aqueous extract of M. oliefera Lam. roots are given in Figure 3 and Table 4.
[Track 1: 4 µl of Ethanol extract; Track 2: 8 µl of Ethanol extract; Track 3: 4 µl Rutin; Track 4: 8 µl Rutin; Track 5: 4 µl of Aqueous extract; Track 6: 8 µl of Aqueous extract]
5.6.1 Anti-diabetic activity of M. oliefera Lam. roots extracts
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Table 4: Evaluation of Moringa oliefera root extracts on for the antidiabetic activity.
|
Sr. No. |
Groups |
Day 1 |
Day 7 |
Day 14 |
Day 21 |
|
1 |
Normal |
91.16±10.36 |
91.34±7.86 |
93.33±7.88 |
90.23±8.74 |
|
2 |
Control |
217±12.03 |
311±14.65 |
291±7.36 |
328±4.5 |
|
3 |
MO Aq. |
186.33±8.22 |
259.66±11.21 |
233±12.01 |
222±11.08 |
|
4 |
MO Alc. |
220.66±5.02 |
205.5±6.34 |
187±12.00 |
150.5±14.5 |
|
5 |
Standard |
234±18.47 |
209±13.46 |
167±17.33 |
89±9.00 |
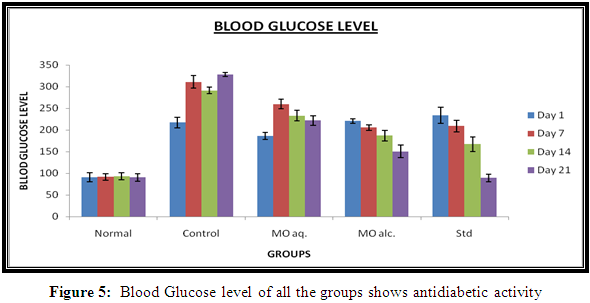 Blood Glocose Level
Blood Glocose Level
MO root extracts had shown antidiabetic effect on STZ-induced diabetic wistar albino rats. MO root extracts had prevented the loss of body weight. MO aqueous extract had shown significant (p ≤0.05) decreases in elevated blood glucose level from (259.66±11.21) to (222±11.08) and MO alcoholic extract had also shown decreases in blood glucose level from (220.66±5.02) to (150.5±14.5) in comparison with Diabetic control group. The results were shown in Table 5.
Diabetic rats exhibited significantly higher glucose level compared to normal rats. Treatment with of MO extracts significantly decreased elevated glucose levels in diabetic rats. The decrease in glucose levels in alcoholic extract of MO shows significant decrease in glucose level compared to aqueous extract exhibited significant(p<0.05) glucose lowering activity. The results were shown in Figure 5.
Table 5: Evaluation of Moringa oliefera root extracts on for the antihyperlipidemic activity.
|
Sr. No. |
Groups |
TC |
HDL-C |
LDL-C |
TG |
LDL/HDL |
TC/HDL |
|
1 |
Normal |
128±9.85 |
36±2.08 |
38.00±5.01 |
81.33±2.9 |
1.8±0.012 |
2.08±0.1 |
|
2 |
Control |
86±7.57 |
17.66±0.88 |
63.00±6.88 |
137.0±4.95 |
2.37±0.05 |
8.59±0.09
|
|
3 |
MO Aq. |
70.33±2.18 |
18.67±2.90 |
32.66±3.84 |
123.2±7.0 |
2.00±0.03 |
3.25±0.2 |
|
4 |
MO Alc. |
85.00±9.16 |
24.33±5.03 |
38.66±1.03 |
108.8±4.8 |
2.20±0.06 |
4.37±0.02 |
|
5 |
Standard |
54±1.73 |
14±0.57 |
24.66±0.57 |
115±6.27 |
1.60±0.065 |
3.09±0.08 |
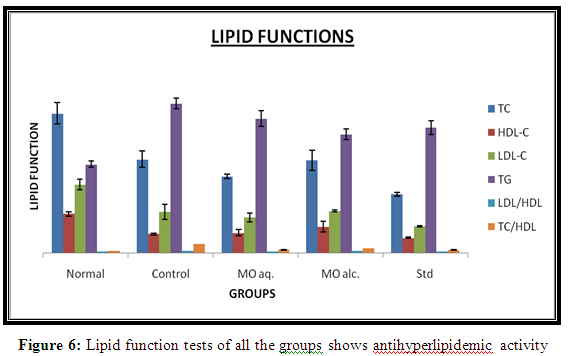 Serum cholesterol
Serum cholesterol
High cholesterol diet rats that are control group exhibited significantly higher cholesterol level in diabetic rats. Treatment with of MO extracts significantly decreased elevated cholesterol levels in hyperlipidemic rats. The decrease in cholesterol levels in aqueous extract (70.33***±2.18) of MO showed significant decrease in cholesterol level compared to alcoholic extract (85.00**±9.16) exhibited significant (p<0.05) cholesterol lowering activity. However, alcoholic extract was also significantly reduced TC when compared with control group (86.0**±7.57). The results were shown in Table 5 and Figure 6.
Serum HDL-Cholesterol
High cholesterol diet rats that are control group (17.66**±0.88) exhibited significantly lower HDL-C levels as compared to normal control rats (36.0±2.08). Treatment with extracts of MO significantly increased HDL-C levels. The rate of increase in HDL-C level in alcoholic extract (24.33*±5.03) was higher compared to aqueous extract (18.67**±2.90) of MO exhibited significant (p<0.001). The results were shown in Table 5 and Figure 6.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Serum LDL-Cholesterol
High cholesterol diet rats that are control group (63.00**±6.88) exhibited significantly high LDL-C levels as compared to normal control rats (38.00±5.01). Treatment with extracts of MO significantly decreased LDL-C levels. The rate of decrease in LDL-C level in aqueous extract (32.66**±3.84) was higher compared to alcoholic extract (38.66**±1.03). The results were shown in Table 5 and Figure 6.
Triglycerides
High cholesterol diet rats that are control group (137.0***±4.95) exhibited significantly high Triglycerides levels as compared to normal control rats (81.33±2.9). Treatment with extracts of MO significantly decreased Triglycerides levels. The rate of decrease in LDL-C level in alcoholic extract (108.8**±4.8) was higher compared to aqueous extract (123.2***±7.0). The results were shown in Table 5 and Figure 6.
LDL/HDL ratio
High cholesterol diet rats that are control group exhibited significantly high HDL ratio as compared to normal control rats (2.37***±0.05). Treatment with extracts of MO significantly decreased serum HDL ratio. The rate of decrease in HDL ratio in aqueous extract (92.00*±0.03) was higher compared to alcoholic extract (2.20***±0.06). The results were shown in Table 5 and Figure 6.
TC/HDL ratio
High cholesterol diet rats exhibited that are control group (8.59***±0.09) significantly high TC/HDL ratio as compared to normal control rats (2.08±0.1). Treatment with extracts of MO significantly decreased serum HDL ratio. The rate of decrease in HDL ratio in aqueous extract (3.25***±0.2) was higher compared to alcoholic extract (4.37***±0.02). The results were shown in Table 5 and Figure 6.
Table 6: Evaluation of Moringa oliefera root extracts on for the measurement of change in liver function.
|
Sr. No. |
Groups |
SGOT |
SGPT |
ALP |
BILIBUBIN |
|
1 |
Normal |
18.5±4.24 |
34±2.03 |
105.67±2.42 |
1.85±0.010 |
|
2 |
Control |
95±10.6 |
88.8±3.24 |
174.45±5.31 |
0.35±0.031 |
|
3 |
MO Aq. |
52.34±8.48 |
49.0±2.78 |
137.5±4.00 |
1.57±0.012 |
|
4 |
MO Alc. |
48.67±4.24 |
68.8±4.242 |
127.5±3.67 |
1.5±0.098 |
|
5 |
Standard |
46.67±7.01 |
45.00±1.16 |
120.43±2.31 |
1.00±0.070 |
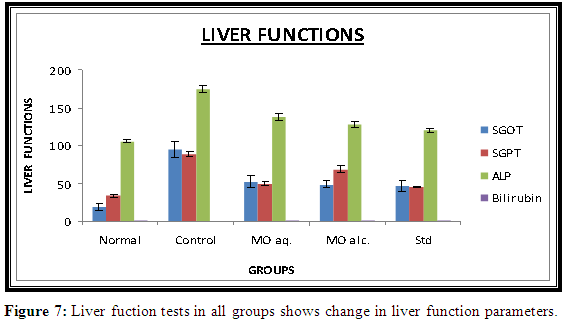
There was rise in serum SGPT (95±10.6), SGOT (88.8±3.24) and ALP (174.45±5.31) level in STZ-induced diabetic rats as compared to normal rats. Alcoholic and aqueous extracts of MO significantly reduced the elevated serum SGPT, SGOT and ALP levels. Both the extracts of MO significantly increase in the Serum bilirubin level. Results are significant at p<0.05 when compared to diabetic control group. The results were shown in Figure 7.
Histopathological changes in Liver of rats
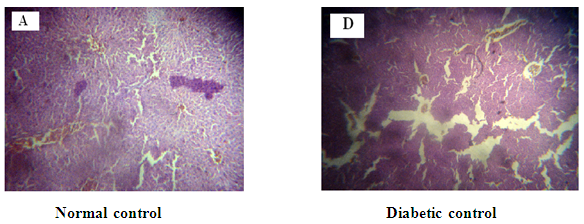
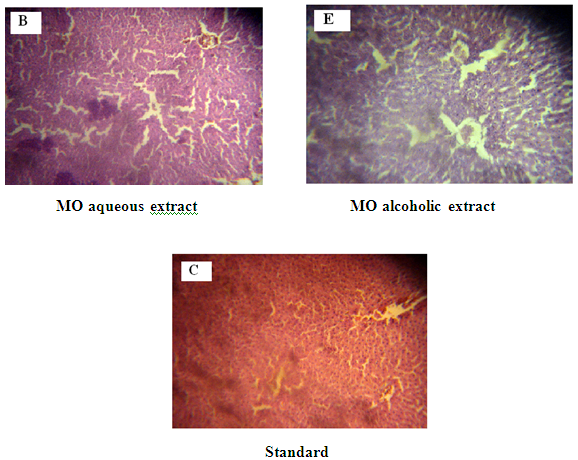
Figure 8: A: Liver from Normal control Group 1 with normal stromal cells.
B: Liver from Aqueous extract Group 3 with healed stromal cells (Partial).
C: Liver from Standard Group 5 with complete healed stromal cells.
D: Liver from diabetic animal Group 2 with inflammatory process in the portal triad and enlarged stroma (arrow).
E: Liver from alcoholic extract Group 4 with necrotic cells.
The liver of the normal control rats presented typical histological organization, matching the description shown in Figure 8A19. In control rats treated with M. oliefera extracts, the liver also displayed typical his¬tological organization, except for an inflammatory process in the stroma and hypertrophic nuclei in some of the he¬patocytes shown in Figure 8B and Figure 8E. In diabetic rats, the hepatocytes were enlarged, with vacuolar cytoplasm and hypertrophic nuclei (arrow). The stroma displayed discreet disorganization with enlargement of the space between the hepatocyte plaques, sinusoidal dilatation, and an infiltrative inflammatory process in the periportal region. These results indicate that the treatment with M. oliefera extracts was unable to prevent these morphological alterations in the liver. Further histopathological and toxicological studies are necessary in order to better evaluate the effects of M. oliefera extracts on the liver. Although morphological changes in alcoholic extract treated group was higher with compared to aqueous extract treated and toxicity in liver was also higher in diabetic treated group shown in Figure 8B.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Histopathological changes in Kidney of rats
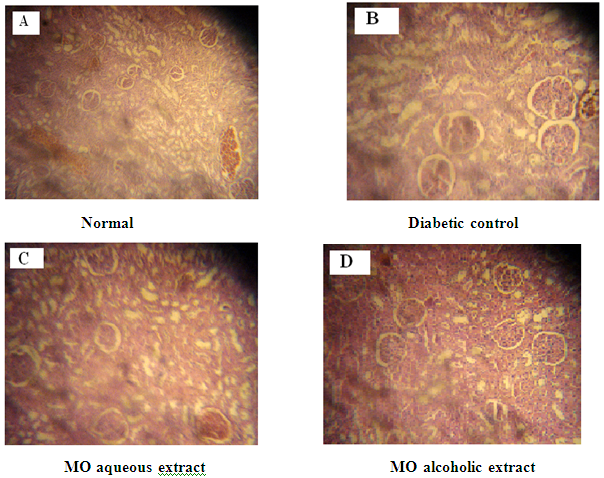
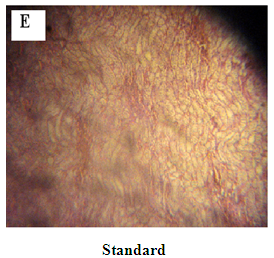
Figure 9: A: Kidney from Normal control Group 1 with normal renal tubules.
B: Kidney from Diabetic rat treated with water displaying altered glomerular morphology, inflammatory process in renal papilla and dilatation of tubules (arrow) group 2.
C: Kidney from Aqueous extract Group 3 with healing renal tubules.
D: Kidney from Alcoholic extract Group 4 with morphological alteration in renal tubules (Partial healing).
E: Kidney from Standard Group 5 with completely healed renal tubules.
In diabetic normal control rats, the kidney presen¬ted a typical histological structure with several morphological changes shown in Figure 9A. In the kidneys of the diabetic rats (Figure 9B), many pathological alterations were found: partial cellular lesion with aci-dophilic material in the glomerulus, concentric fibrosis with distortion of the tubular wall and enlargement of the tubular lumen, vacuolization of the cyto¬plasm of the tubular epithelium cells; atypical nuclei, and inflammatory processes in the interstitial spaces in renal papilla and pelvis regions. These alterations are typical of diabetic nephropathy20. The diabetic rats treated with M. oliefera extracts displayed no glomerular or tubular pathological alterations, although slight changes in morphology were seen. Inflammatory processes were absent, but fibrotic scars were found at somewhere shown in Figure 9C and Figure 9D. These findings suggest that treat¬ment with M. oliefera may have an anti-inflammatory effect on the kidneys.
DISCUSSIONS
The present study was aimed to evaluate the antidiabetic and antihyperlipidemic activity of aqueous and alcoholic extracts of MO roots in diabetic rats. Blood glucose level was lowered with both the extracts, when it was compared with diabetic control. Standard drug Glipizide also optimally reduced the blood glucose level against diabetic rats.
Both the extracts (aqueous and alcoholic) reduced the TC, LDL, TG, HDL/LDL, TC/LDL compared with diabetic control group. As far as HDL which is responsible having protective action on heart, was also increased with both the extracts compared with control. Bilirubin concentration was increased with both the extracts in diabetic rats compared with diabetic control group. As it was evident that diabetic control rats shown decrease in bilirubin concentration.
Further, the intention was towards establishing the toxicity studies especially on liver and kidney. From the study, it is now evident that aqueous extract of MO was highly effective comparative to alcoholic extract. Standard drug Glipizide shown better results compared with diabetic control without producing toxicity on liver and kidney. From the histopathological study, no doubt we can say that alcoholic exract was somewhat toxic on liver and kidney, whereas results of aqueous extract found to be better except slight alterations in the morphological structure in kidney.
The antidiabetic and antihyperlipidemic activity afforded may be due to the presence of active constituents like alkaloids moringine and moringinine. Regarding the toxicity studies, still there is needed to carry out further studies for both the extracts of MO roots.
CONCLUSION
In conclusion, alcoholic and aqueous extract of MO had shown antidiabetic activity in STZ-induced diabetic rats with reference to various parameters such as Body weight, Blood glucose, Serum cholesterol, Serum triglyceride, Serum HDL, Serum LDL, LDL/HDL, TC/HDL, Serum SGPT, Serum SGOT, Serum ALP, Serum bilirubin. So, in the present study, STZ was able to induce typical signs of diabetes, such as weight reduction, polyphagia, polydypsia, polyuria, hyperglycemia, reduced hepatic glycogen levels. Treatment of the STZ treated rats with M. oliefera extracts significantly reduced their glycemic levels, restored hepatic glycogen levels, and produced favorable histopathological results. Moreover, the decoction of M. oliefera had no diuretic effect in the control or diabetic groups, where diuresis is a common side effect of many teas and herbal infusions.
ACKNOWLEDGMENT
The authors are grateful to Management Trustee, Vidyabharti Trust for the financial support and encouragement in carrying out this project.
REFERENCES
1. Kirtikar KR and Basu BD. Indian Medicinal Plants. (M/s Bishen Singh, Mahendra Pal Singh, New Cannaught Place, Dehra Dun), 2nd Edn, Reprint, 1975, 35(1), pp. 676-683.
2. Fuglie LJ. New Uses of Moringa Studied in Nicaragua. ECHO Development Notes #68, June, 2000
3. Fahey JW, AT Zalcmann, and P Talalay. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry, 2000; 56(1): 5-51.
4. Kalkunte S, Swamy N, Dizon DS, Brard L. Benzyl isothiocyanate (BITC) induces apoptosis in ovarian cancer cells in vitro. J Exp Ther Oncol., 2006; 5: 287–300.
5. Satyan KS, Swamy N, Dizon DS, Singh R, Granai CO, Brard L. Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: role of caspase and MAPK activation. Gynecol Oncol., 2006; 103:261–270.
6. Dahot MU. Antimicrobial activity of small protein of Moringa oliefera leaves. Journal of Islamic Academy of Sciences 1998; 11(1): 27-32.
7. Gupta M. Mazumder UK, Chakrabarti S. CNS activities of methanolic extract of Moringa oliefera root in mice. Fitoterapia., 1999; 70: 244-250.
8. Guevara AP, Vargas C, Sakurai H, Fujiwara Y, Hashimoto K, Maoka T, et al. An anti-tumor promoter from Moringa oliefera Lam. Mutation Res., 1999; 440(2): 181-188.
9. Lalas S and Tsaknis J. Extraction and identification of natural antioxidant from the seeds of the Moringa oliefera tree variety of Malawi J Am Oil Chemists Soc., 2002; 79(7): 677-683.
10. Ray K, Hazra R and Guha D. Central inhibitory effect of Moringa oliefera root. extract, Possible role of neurotransmitters, Indian J Exp Biol., 2003; 41(11): 1279-1284.
11. Ruckmani K, Kacimani S, Anandan R and Jayker B. Effect of Moringa oliefera Lam. on Paracetamol - induced hepatotoxicity, Indian J Pharm Sci., 1998; 60(1): 33-35.
12. The Wealth of India- A Dictionary of raw materials, Publications and information Directorate,CSIR, New Delhi, 1956, pp. 35-36.
13. Harbone JB. Phytochemical Method, Chapman Hall, 1988; pp. 117-119.
14. Mohammed A. Text Book of Pharmacognosy, CBS Publishers and Distributors, 1994; pp. 81- 447.
15. Agrawal OP. Advanced practical organic chemistry, Goel Publishing House, 2000; pp. 43-59.
16. Divakar MC. Plant Drug Evaluation, CD Remedies Publication, 2003; pp. 49-89.
17. S. Kumar, V. Kumar, Prakash O. Antidiabetic and hypolipidemic activities Of Hibiscus Tiliaceus (L.) flowers extract in Streptozotocin induced diabetic rats. Pharmacologyonline, 2010; 2: 1037-1044.
18. Chatterji S, Singh RK, Shukla S, Yadav DK, Watal G. Glycemic effect of freeze dried Murraya koenigii - evidence based study. International Journal Of Pharma And Bio Sciences, 2010; 1(2): 1-9.
19. Teckman, JH, Loethen JK, Perlmutter DH. Fasting in a1-antitrypsin deficient liver: constitutive activation of autophagy. Am. J. Physiol., 2002; 263: G1156-G1165.
20. Thorup C, Ollerstam A, Persson EG, Torffvit O. Increased tubule glomerular feedback reactivity is associated with increased NO production in the Streptozotocin-diabetic rat. J. Diabetes Compl., 2000; 14: 46-52.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org









