 About Author:
About Author:
Mr. Jagmohan Rai Agarwal,
M.Pharm (1968), Industrial experience SSI sector, nearly 37 years, retired from own Industry,
Founder President of M.P.Pharmacy Graduates’ Association,
President: M.P.Pharmaceutical Manufacturers’ Organisation,
Founder President : M.P. Small Scale Drug Manufacturers’ Association,
President Indian Pharmaceutical Association, M.P. State Branch, Indore,
Recently submitted thesis for award of Ph.D. on title “Enforcement of Drug Laws-Globalization vis-à-vis Indian Drug Laws”
(Email: sharda_jollo@yahoo.co.in)
Quality of Pharmaceuticals has been a concern of the World Health Organisation (WHO) since its inception. The setting of global standards is requested in Article 2 of the WHO constitution which sites as one of the organisation’s functions that it should develop, establish and promote International standards with respect to food, biological, pharmaceuticals and similar products.
Campbell (US FDA) was the first leading architect of the present Federal Food, Drug and Cosmetics Act passed in 1938. He differed sharply with ‘Willey’ in his belief that Court proceedings were not the only proper way to secure compliance.
[adsense:336x280:8701650588]
A simple broad definition of “regulation” is ‘the use of public authority to set and apply rules and standards’ in an attempt to “manipulate prices, quantities ( and distribution ), and quality of products” These types of legal and administrative restrictions are only fully successful in the context of a well-resourced regulatory framework e.g. both for implementation and monitoring as well as the existence of a well-functioning judicial system (for enforcement and sanctioning). Governmental regulation is inherently a creature of the political process so that changes at the level of a drug regulatory authority often require difficult negotiations.
In 1911 U. S. v/s Johnson, the Supreme Court ruled that the 1906 Food and Drugs Act does not prohibit false therapeutic claims but only false and misleading statements about the ingredients or identity of a drug, this ruling was overcome by Sherley amendment in 1912. In 1930 the name food, drug and insecticide Administration shortened to Food and Drug Administration (FDA)
1937 Elixir of Sulphonamide containing poisonous solvent diethyl glycol kills 107 persons, dramatizing the need to establish drug safety before marketing and to enact the pending Food and Drug Law.
The Federal Food, Drug and Cosmetics (FDC) Act passed in 1938 and Walter G. Campbell was appointed the first Commissioner of Food and Drug. In the year 1962, after ‘Thalidomide’, a sleeping pill, tragedy, found to cause birth defects in Western Europe, the drug manufacturers were required to prove to FDA the effectiveness of their products before marketing them.
A regulation is neither efficient nor effective if it is not complied with or can not be effectively enforced. A policy instrument that appears effective may be difficult to implement. Indeed, many countries may promulgate regulations that go beyond the capacity of their compliance and enforcement capabilities. For developing countries, simpler operational structures for drug registration and quality control testing of pharmaceuticals are likely to be more appropriate than complex structures and therefore, ‘appropriate regulatory’ technology is strongly recommended. It should be the goal of any developing country to create a pharmaceutical regulatory administrative system that is transparent, not overly legalistic, not too expensive to administer, that can not be easily manipulated, either from within or without, and that has regulations that can actually be enforced. Therefore, Drug Regulatory Authorities of developed countries are not necessarily models to emulate in the short (or even the long) term. For instance, legal sanctions such as barring a product from being sold or advertised that can be sued by the U.S. FDA may not be feasible in developing countries that lack regulatory and legal enforcement
The standards for drug review and approval in U.S. are the Best in the world and the safety of drug supply mirrors these high standards.
[adsense:468x15:2204050025]
In Australia Regulatory framework is based on a risk management approach designed to ensure public health and safety and while at the same time freeing Industry from any unnecessary regulatory burden. TGA has developed constructive partnership with Industry
In developed countries and some of the developing countries civil/financial penalties instead of criminal prosecution is preferred for violation of Law. Whereas in India law is being enforced under the threat of Departmental action (Suspension or Cancellation of Licences) and/or criminal prosecutions. The basis of all these actions is test reports of Government laboratories either under provincial or Central Government control. The status of these laboratories, in majority cases, is not better than a mismanaged grocery/ kirana shop. The concerned Regulatory Authority prefers to keep himself free of controversies and allegations of not taking action which results in a short cut by proposing either departmental or criminal prosecution and shoulder is changed.
Availability of Safe, Efficacious and Quality drug formulations at affordable price is the centre point of ‘Regulatory Laws’ in the world. The degree of its ENFORCEMENT and PENALTIES for violations differs from country to country. Misbranded, Adulterated, Spurious/Counterfeit, Not of Standard Quality/Sub potent medicines and high prices are mainly due to improper enforcement of Regulatory Laws.
There are many ways to write FDA’s history. It can be a story of laws which Governments’ enact, a story of famous cases to enforce those laws, a story of the organization and the people who built it, or a story about the changing technology and the scientific controversies, some settled others still unsolved. And it can be all of these but not in a few pages.
If there is one dominating theme it is the change from a law, that was primarily a criminal statute protecting customers through the deterrent effect of court proceedings to a law that is now dominantly through informative regulations and pre-market controls.
India is probably the only country in the world where a State Regulatory Association filed a petition before High Court seeking direction to the State Government to fill up the vacancies of regulatory officials in the department. India is again probably the only country where the Government and/ or Regulatory Authorities are clearly violating provisions of the ‘Act’ and ‘Rules’ without any accountability.
To make the provisions of Law effective, it’s proper, timely and unbiased Enforcement must be the only motto. Making laws more and more stringent does not serve the purpose. The recent amendment ( year 2009) in the Indian Drugs and Cosmetics Act, enhancing punishments to violators of the Law, is like “handing over loaded machine guns in the hands of monkeys”, commented an Ex Head of a State Drug Regulatory Department. Like China, let there be accountability of Enforcement Agency especially in India.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
As per WHO, National Governments are responsible for establishing strong National Drug Regulatory Authorities (DRAs) with clear mission, solid legal basis, realistic objectives, appropriate organizational structure, adequate number of qualified staff, sustainable financing, access to technical literature, equipment and information, capacity to exert effective market control, DRAs’ must be accountable to both, the Government and the public and their decision making processes should be transparent. Monitoring and evaluation mechanisms should be built into regulatory system to access attainment of established objectives.
As on today the Indian Drug Laws are under revolutionary changes, for the last over about 10 years the licensing to manufacture for certain category of Drugs and Pharmaceuticals have been assigned to Central Drugs Standard Control Organization (CDSCO) in DCGI office, the provisions of ‘New Drugs’, Banning of irrational FDC etc. have been introduced / widened. The Government of India is also in process to form National Drug Authority, desiring (?) it to be at par with US FDA and before that squeezing of powers of State FDA on various counts is in pipeline.
The trend of transfer of power from States/UTs to CDSCO, as for as related to licensing to manufacture drugs is clearly seen from the year 1996 when power to license to manufacture Large Volume Parentrals were shifted from States to CDSCO. It continued with widening of criteria of ‘new drug’, though public does not know claims approved. This power game is on enlargement to formation of proposed National Drug Authority (NDA) under the shadow of uniform implementation of the ‘Act’ and ‘Rules’. Though the Licensing/Approving powers in respect of some of the categories are vested with the Central Drug Regulatory is not uniformly implemented and it is also a party to violation of present Drug Laws.
The Indian regulatory system is nowhere close to the sophisticated regulatory systems of developed nations, and because the enforcement of legislation is much poor than internationally accepted norms, the entry barrier for developed nations into the Indian market is practically non-existent.
The U S Supreme Court upholds the 1962 drug effectiveness law and endorses FDA action to control entire classes of products by regulations rather than to rely only on time-consuming litigation. Fines Enhancement Laws of 1984 and 1987 amend the U S code to greatly increase penalties for all federal offences.
In India the Private Commercial Drug Testing laboratories are subjected to licensing and periodic audit by Regulatory Authorities, but Government Testing Laboratories both at Central and State level, are virtually exempted (?) from such audit. It is pertinent to highlight that test reports of these Government Laboratories, if adverse, form the basis of initiating departmental and/or criminal action against the concerned under the provisions of Law.
Let these Government Laboratories, including the CDL/CDTL be subjected to audit by outside private competent agencies and laboratories qualify may be authorized to test and issue test reports for that particular class of drug(s) and/or cosmetic(s) for which it has been found equipped and supported by well trained expert staff. This practice be continued every year. These qualified labs. be notified & Test Reports issued by such laboratories for approved class of drug(s) and/or cosmetic(s) be only considered valid under the ‘Act’/Rules.
In India, the Drug Enforcement Laws are under severe review and further major amendments are awaited in near future. After 1982, various provisions of Drugs & Cosmetics Act 1940 and Rules 1945 made there under are either already amended or widened. The import registration is brought into and the license fee to manufacture for sale and also for sale domestically is increased exorbitantly, the list of banned fixed dose combinations is exhaustive, the cGMP as schedule M is revised. More so new stringent penal provisions, minor financial penalties and compensation (first time) have been notified in the Drugs and Cosmetics (Amendment) Act 2009.(www.cdsco.nic.in)
In India Medium and Small Pharma Industrial units have been the target of MNC’s and LSI with an aim to exclude them from the business. To achieve this goal various gimmicks, illustrated below, were adopted, since the year 1999, in corroboration with those in power:
1. Marketing of Generics by MNCs’ and LSI
2. Incorporation of irrational eligibility criteria for participation in Government Tenders for procurement of medicines viz i) Requirement of WHO GMP certification, which though ultimately turned down by Hon’ble Supreme Court.still forms part of few domestic tender conditions; And ii) Minimum Annual Turn over of ranging from Rs. 1.0 cr. to 50.0 cr.
3. Enhancement of licence fee from few thousand to few lacks
4. Enforcement of Revised Schedule ‘M’
5. Notification of Dr. Mashelkar committee, without proper and effective SSI representation and with pre-determined end result.
6. Manipulation/ Highlighting Test Reports of Govt. Analysts
7. Creation of Interstate business rivalry
8. Levy of Excise duty on Maximum Retail Price
9. Creation of Excise/Tax Free Zones
10.Notification of Amendment to Drugs and Cosmetics Act, based on recommendations of Mashelkar Committee Report, incorporating stringent penal provisions such as: i) enhancement of punishments u/s 27 of the ‘Act’; ii) section 32 any gazetted officer of Central or State Governments authorized in writing could also prosecute ; iii) section 36AC (1) (a): offence relating to adulterated or spurious drug and punishable under clauses (a) and (c) of sub section (1) of section 13, clause (a) of sub section (2) of section 13, sub section (3) of section 22, clause (a) and (c0 of section 27, section 28, section 28A, section 28B and sub sections (1) and (2) of section 30 and other offences relating to adulterated drugs or spurious drugs, made cognizable (iv) section 36 AC (b): Offences cognizable u/s 36AC(a) are made non bailable. (offending sections being reproduced below)
As a result of continuous follow up by the Industry, particularly SME sector, the Government of India in the Ministry of Health issued Directive to States/UTI’s under section 33-P of the ‘Act’ as “Guidelines for taking action on samples of drugs declared spurious or Not of Standard Quality in the light of enhanced penalties under the Drugs and Cosmetics (Amendment) Act, 2008”
11.Enforcement of “Good Laboratory Practices” (Excluding the Govt Testing labs and CDL/CDTL)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Section 13 (1): Whoever himself or by any other person on his behalf imports:
(a) any drug deemed to be adulterated under section 9A or deemed to be a spurious drug under section 9B or any spurious cosmetics referred to in section 9D or any cosmetic of the nature referred to in clause (ee) of section 10 shall be punishable with imprisonment for a term which may extend to three years and fine which extend to Five thousand rupees.
(c ) any drug or cosmetic in contravention of the provisions of any notification issued under section 10A, shall be punishable with imprisonment for a term which may extend to three years, or with fine which may extend to ten thousand rupees, or with both.
(2) Whoever having been convicted of an offence –
(a) under clause (a) or clause (c) of sub section (1), is again convicted of an offence under that clause, shall be punishable with imprisonment for a term which may extend to five years, or with fine which may extend to ten thousand rupees, or with both.
Section 22 (3)If any person willfully obstructs an Inspector in the exercise of the powers conferred upon him by or under this Chapter, 2[or refuses to produce any record, register or other document when so required under clause (cca) of sub-section (1),] he shall be punishable with imprisonment which may extend to three years, or with fine, or with both.
Section 27: Penalty for manufacture, sale, etc., of drugs in contravention of this Chapter.—whoever, himself or by any other person on his behalf, manufactures for sale or for distribution, or sells, or stocks or exhibits or offers for sale or distributes,
(a) any drug deemed to be adulterated under section 17A or spurious under section 17B and which when used by any person for or in the diagnosis, treatment, mitigation, or prevention of any disease or disorder is likely to cause his death or is likely to cause such harm on his body as would amount to grievous hurt within the meaning of section 320 of the Indian Penal Code (45 of 1860), solely on account of such drug being adulterated or spurious or not of standard quality, as the case may be, shall be punishable with imprisonment for a term which shall not be less than ten years but which may extend to imprisonment for life shall also be liable to fine which shall not be less than ten lakh rupees or three time value of the drugs confiscated whichever is more;
(c) any drug deemed to be spurious under section 17B, but not being a drug referred to in clause (a) shall be punishable with imprisonment for a term which shall not be less than seven years but which may extend to imprisonment for life and with fine which shall not be less than three lakhs rupees or three time the value of the drugs confiscated which ever is more.
Provided that the Court may, for any adequate and special reasons, to be recorded in the judgment, impose a sentence of imprisonment for a term of less than seven years but not less than three years and of fine of not less than one lakh rupees.
28. Penalty for non-disclosure of the name of the manufacturer, etc.—Whoever contravenes the provisions of section 18A or section 24 shall be punishable with imprisonment for a term which may extend to one year, or with fine which shall not be less than twenty thousand rupees or with both.
28A. Penalty for not keeping documents, etc., and for non-disclosure of information.—Whoever without reasonable cause or excuse, contravenes the provisions of section 18B shall be punishable with imprisonment for a term which may extend to one year or with fine which may extend to one thousand rupees or with both.
28B. Penalty for manufacture, etc., of drugs or cosmetics in contravention of section 26A.—Whoever himself or by any other person on his behalf manufactures or sells or distributes any drug or cosmetic in contravention of the provisions of any notification issued under section 26A, shall be punishable with imprisonment for a term which may extend to three years and shall also be liable to fine whichshall not be less than twenty thousand rupees or with both..
30. Penalty for subsequent offences.—(1) whoever having been convicted of an offence-
(a) under clause (b) of section 27 is again convicted of an offence under that clause, shall be punishable with imprisonment for a term which shall not be less than seven years but which may extend to ten years and with fine which shall not be less than two lakh rupees:
Provided that the Court may, for any adequate and special reasons to be mentioned in the judgment, impose a sentence of imprisonment for a term of less than seven years and of fine of less than one lakh rupees;
(b) under clause (c) of section 27, is again convicted of an offence under that clause shall be punishable with imprisonment for a term which shall not be less than ten years but which may extend to imprisonment for life and with fine which shall not be less than fifty thousand rupees;
(c) under clause (d) of section 27, is again convicted of an offence under that clause shall be punishable with imprisonment for a term which shall not be less than two years but which may extend to four years or with fine which shall not be less than five thousand rupees, or with both.
(2) Whoever, having been convicted of an offence under section 29 is again convicted of an offence under the same section shall be punishable with imprisonment which may extend to two years or with fine which shall not be less than ten thousand rupees or with both.
The authentic data in respect of availability of Spurious and Not of Standard Quality Drugs in the country.
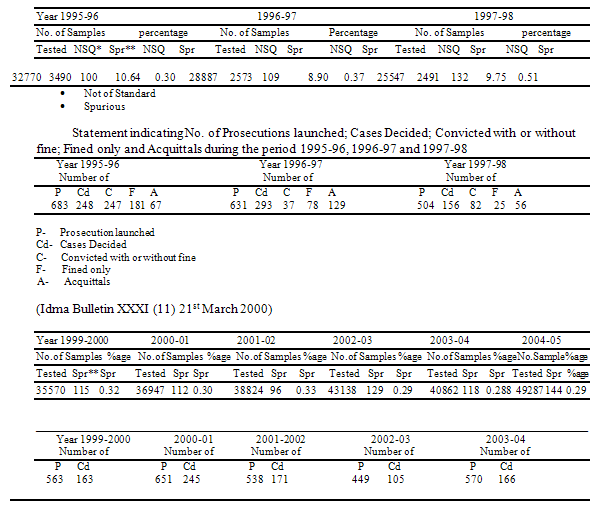
The author is reminded of a true story of Madhya Pradesh:
A product ‘Benzyl Benzoate Application’ was declared grossly Not of Standard quality in as much as the Assay was reported only 10% of claim. The prosecution was ordered by the then Controller (IAS) and the proprietor suffered a heart attack. A group of persons representing the association explained the entire situation to the controller and he was convinced to get the sealed sample tested a fresh by another Govt. Analyst in his presence. The drug was found to conform the prescribed standards and the Controller being dashing and honest immediately withdrew the prosecution permission, before it was launched.
It also reminds the author of one more instance which goes to demonstrate the techno- legal IQ of officers even of the cadre of Drugs Controller. A drug was reported NSQ by the State Govt. Analyst only because of improper storage in the costal area of the country. On being questioned the Drugs Controller sharply replied that the manufacturer should ensure that the drug formulation stands the hot and humid climate of the State.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Yet another blast appeared in ‘Times of India’ and carried over by www.drugscontrol.org 30.12.2009 : In a unique modus operandi Mastermind Navneet Sharma and his gang use to sign contracts with MNCs’ for exposing counterfeit drugs of these companies in the market. These persons used to charge up to Rs. 1.25 lakhs from each company for a police raid. They used to plant the counterfeit medicines prepared by these companies at a place and then they, themselves, informed the police and took them to that place. After the police recovered the drugs, the accused would send a copy of FIR to the companies and earn the contract money. Obviously the object was to malign SSI sector and to throw them out of business.
Similarly following instances would demonstrate that many a times reports issued by either Government Analysts and/or Director Central Drug Laboratory on the face thereof can not be treated as reliable even than the prosecution is often faced by the licencees:-
1. Syrup Promethazine when stored under sun light, promethazine converts in its derivative which does give not positive results on being subjected to test for promethazine as per IP- The drug is either declared grossly Not of Standard or Spurious.
2. A drug formulation is reported Not of Standard Quality in respect of ‘Description’ when as per General Notices of IP ‘Description’ is not a part of Standards when not mentioned below the heading ‘Standards’ in the monograph.
3. A report issued by the Director, CDL, u/s 25(4) of the ‘Act’ in respect of a P or P medicine where all the parameters were found to comply with prescribed standards but instead of “is not” the words “is” was cut on report and thus the drug was reported as NSQ though all the parameters were found complying. Ultimately on persuasion a ‘Corrigendum’ was issued by the Director and concerned licencee was discharged from the criminal complaint after about more than three years.
4. Declaring the drug as NSQ on the basis of insufficient quantity of sample available for testing.
5. Adopting of own method of analysis in respect of P or P medicine even though the licencee supplied the method when asked for.
6. Adopting of method other than prescribed in relevant Pharmacopoea due to non availability of required instrument and declaring the drug as NSQ.
The regulatory agency, office of the Drugs Controller General of India, admits it is ill-equipped to handle the extent of vigilance required to comb a vast country with just insufficient drugs inspector at the central and state level. The All India Drugs Control Officers’ Confederation (AIDCOC), a representative body of the drugs inspectors, estimates that India needs at least 4500 additional drugs inspectors to monitor 15000 drug manufacturing units and more than half a million retail outlets. The drug control regime is “crippled”, says Ravi Udai Bhaskar, AIDCOC secretary general. “There is inadequate manpower, infrastructure and auxiliary staff. This issue of up gradation has been in limbo for 25 years now” he says, but bristles at the notion that drug inspectors, sometimes suspected of being part of the problem, are also to blame for the spread of counterfeit drugs.
As per media report Central and a few State Governments have proposed to hire Drug Inspectors on contract basis. Fantastic Idea, but why not to invite International tenders for uniform implementation of drug laws in the country and that the winning agency may be allowed to enter into subcontracts for States’.
The Mashelkar committee while recommending for stringent penal provisions in the ‘Act’ observed many remarkable short comings in the implementation of provisions of existing ‘Act, viz. inadequate or weak drug control infrastructure at the State and Central level, inadequate testing facilities, shortage of drug inspectors, non-uniformity of enforcement, lack of specially trained cadres for specific regulatory areas, non-existence of data bank and non availability of accurate information. The committee further observed out of information received from 31 States/UTs, only 7 drug testing laboratories were reasonably equipped/staffed.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The committee noted (on the basis of data pertaining to on or before the year 2003) that only about 36000 samples, which is about 1% of batches of drugs manufactured in the country, are exposed to scrutiny by government testing labs. The limitations in testing of drug samples in the government labs. are related to the absence or lack of sophisticated instruments, lack of trained analysts, lack of commitment, lack of reagents, non-validated methods, shortage of funds, inadequate number of staff and in many cases a combination of more than one of these constraints are responsible for the poor state of affairs in these government testing labs.
For the last couple of years it has become a trend/practice to prefer launching criminal prosecution by drug inspectors solely on the basis of a test report of the government analyst, reporting concerned batch to NSQ, without any investigation whatsoever, without application of techno-legal mind, without jurisdiction in the state of sampling. Collection of records related to manufacturing, testing, sale, names/addresses of directors/partners/expert staff amounts to investigation by these so called Inspectors/ other senior officials for putting criminal case against accused persons who happen to be pharma manufacturers.
The allegation in majority of these cases is violation of sec. 18(a)(i) punishable u/s 27(d) of the ‘Act’. The author is of the firm opinion that the circumstances under which violation u/s 18(a)(i) is alleged and punishment u/s 27(d) sought is not tenable in law. This topic in it self deserves to be dealt in a separate article.
A recent incidence occurred at Umaid Hospital, Jodhpur, where in some female patients died and the allegation is conveniently levied on the I.V. fluid being contaminated on the basis of the report of an unauthorised laboratory selected by the hospital and without following the mandatory procedure prescribed under the ‘Act’
The Medical Superintendent of the Hospital made a complaint to the police and the FIR is registered u/s 328 IPC, the said IPC provision is reproduced here below:
Section 328 IPC: Causing hurt by means of poison, etc. with intent to commit an offence-“Whoever administers to or causes to be taken by any person any poison or any stupefying, intoxicating or unwholesome drug or other thing with intent to cause hurt to such person or with intent to commit or to facilitate the commission of an offence or knowing it to be likely that he will thereby cause hurt, shall be punished with imprisonment or either description for a term which may extend to ten years, and shall also be liable to fine”
Based on above manipulated and politically motivated facts a person said to be Manager of the Pharmaceutical manufacturing company was immediately arrested and is awaiting bail for the last nearly two months.
Interestingly hundreds of samples of I.V. fluid manufactured by the involved company were drawn for test/analysis and the Apex lab. i.e. Central Drug Laboratory, Calcutta has reported all these batches, to conform to prescribed standards. Samples of disputed batch were drawn from different places, where supplies made, which are reported as of Standard Quality except one sample drawn from the Hospital.
As per the information available the manufacturing facility of the company was thoroughly inspected by State as well as Central Drug Authorities and that no major deficiency is noticed. The entire episode is nothing but trial by media, which should not be permitted.
Misuse of provisions of IPC is bound to cause irreparable loss and injury to the Pharmaceutical Industry, irrespective of its class or category and it could be made an easy target.
Relief to Pharma Industry: The Allahabad Bench of the Uttar Pradesh High Court has quashed the U.P.Government’s controversial order of May 11, 2010 in which the State Government had directed the state police department to initiate criminal proceedings under sections 274, 275 and 276 of IPC against the Drug and Food companies which are found to be marketing sub standard drugs.
Attention of readers is drawn to provisions of newly inserted section 36AC which makes specified offences as cognizable and non bailable.
As per recent guidelines issued u/s 33-P of the ‘Act’, The actions are normally initiated on the basis of test reports of Government Analysts declaring the drug samples as NSQ. One may view an incident that a drug sample is declared NSQ conclusively and a prosecution is launched, ignoring these guidelines, against the company/its directors-partners/expert staff u/s 27(a) or 27(c) r/w sec. 36AC- What will happen?
All the accused persons, irrespective of class and/or category of the industry, shall first be sent behind bars till the bail is granted by the competent Court of Law as per the procedure/provision laid down. The accused person(s) later on may prove the charges as baseless but the damage and the irreparable loss is already done.
It will be premature to comment on what shape the new provisions read with guidelines shall take in time to come. But on the strength of past experience, very attitude of regulatory in the country, negative approach of the media, prejudicial/corrupt thought of politicians and all others concerned, it can be safely inferred that the Journey of PHARMA sooner or later irrespective of class and/or category, is bound to end in JAIL.
In view of amended section 32, apart from Inspector and other persons, power to prosecute has also been vested:
“ Any gazetted officer of the Central Government or a State Government authorized in writing in this behalf by the Central Government or a State Government by a general or special order made in this behalf by that Government”;
More so the offence which was earlier to be tried by Metropolitan Magistrate or JMFC, is now to be tried by the Sessions.
Dr. Eric D Kupferberg, Associate Director, Harvard School of Public Health, Boston, USA in an interview with ‘Pharmabiz’- Vagueness and doubts about laws in implementation have become source of conflicts between the industry and the regulatory affairs in India. The laws should be made for cooperation between the industry and the regulatory authorities. It seems that enforcement of drug laws faces complicated problems in India and it often becomes headache for the regulatory agency and the DCGI is forced to interpret the laws very often.
In certain areas of implementation this conflict exists in U S also, but there is transparency and cooperation in most sides. In the government-industry relation side, India needs cooperation. To create a friendly atmosphere, the enforcement agencies must train their staff in such a way.
According to him US FDA is not acting like policeman and its activities are transparent. It is cooperating with the industry and is made industry friendly. It really helps the drug companies to work with the FDA as they have many informal meetings.(Pharmabiz.com 25.11.2010)
Notification of DCC Minutes: Drugs Consultative Committee (DCC) is constituted under Section 7 of the ‘Act’ by the Central Government, to advise the Central Government, the State Governments and the Drugs Technical Advisory Board on any matter tending to secure uniformity throughout India in the Administration of the Act. Representatives from Central as well as State Governments constitute the Committee.
More than about forty meetings of the ‘DCC’ have taken place till date. Minutes of all these meetings are drawn and sent to all the representatives across the country. In view of the objective with which the DCC is constituted and also in view of the direction given to State Governments U/s 33-P of the ‘Act’ on guidelines in respect of “Spurious Drug Act”, it would be desirable if all the previous and future DCC minutes are posted on the CDSCO site and necessary direction U/s 33-P to State Governments are given.
Discussion on Guidelines to ‘Spurious Drug Act’:
· Directions U/s 33-P whether would be treated as mandatory by State Drug Authorities and the Judiciary.
· When bottom line of the test report is only material which is normally not subjected to scrutiny then why Drug Inspectors with prescribed qualification/experience should be required.
· The meaning of ‘INVESTIGATION’ of a case has got to be taught to Regulatory officials in general.
· Regulatory officials must be trained, updated and be subjected to regular interaction with the Industry and Trade.
· The words ‘criminal intent’ has been used for the first time must be given utmost importance.
· Departmental and penal actions, if any, proposed must be with fairness as desired of from quasi-judicial authority.
· Para 6 of guidelines: Section 36AC which makes certain offences under the Act cognizable and non-bailable has been inserted to facilitate the arrest of anti-social elements involved in the manufacture of spurious or adulterated drugs. The section should therefore be invoked with utmost care and only in cases where it is justifiably felt that it is essential to book the culprits for proper investigations in the case.
· The composition of screening committee should include a senior person from the Bar and who should be nominated as the ‘Chairperson’ of the committee.
· Written permission from the Controlling Authority to prosecute is now precondition.
· The fundamental issue is – Poor state of affairs in most of the Government Laboratories and to some extent in CDL/CDTL, based on whose test reports departmental/criminal actions are proposed/launched.
· Para 13 of the guidelines: For combating the menace of spurious / adulterated drugs a robust infrastructure is essential to implement the provisions of the Drugs and Cosmetics Act. The Drugs Control organization in the States (as well as Centre) are therefore, needed to be strengthened by providing additional manpower, infrastructure, technical capabilities and financial resources for having continuous vigilance about the quality of drugs moving in the market.
It will be like expecting too much from the regulatory, which is headed by non-qualified person(s), in a few States, which is involved in executing many acts contrary to the Law.
The purpose of this Article is not to show an Horror film but to awaken the Pharma Industry as a whole and motivate them to fight out the menace of ‘Spurious Drug Act’ notified in August 2009 at all available forum with full strength in the spirit of ‘ Do Or Die’ or else GOD is great and “Mera Bharat Mahan”.
An appeaser is one who feeds a crocodile, hoping it will eat him last. -Winston Churchill
Power Corrupts. Absolute power corrupts absolutely. -Lord Acton
Success is never found. Failure is never fatal. Courage is the only thing. -Winston Churchill
The time to repair a roof is when the sun is shining. -John F. Kennedy
A house divided against it cannot stand." -Abraham Lincoln
Actually it is befitting the old story ‘person(s) who dug the pit for others fell themselves therein’. The MNCs’ and LSI under whose untiring efforts the “Spurious Drug Act” came into existence through recommendations of Mashelkar Committee and now it is not far when those person(s) are bound to fell in the same pit.
The communication gap between Regulatory and Industry/Trade can be abridged with regular interaction with open mind and that former undertakes treatment of “Egomycin Capsules” 1 cap T.D.S. for five days before each interaction meeting.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









