 About Author:
About Author:
swati khandelwal
Maharishi arvind college of pharmacy,
rajasthan university of health science, jaipur
imswatikhandelwal@gmail.com
1. DIABETES
1.1 INTRODUCTION-
Diabetes is a condition in which the body does not produce enough, or properly respond to, insulin, a hormone produced in the pancreas. Insulin enables cells to absorb glucose in order to turn it into energy. In diabetes, the body either does not respond properly to its own insulin or does not make enough insulin, or both. This causes glucose to accumulate in the blood, often leading to various complications.
The term diabetes, without qualification, usually refers to diabetes mellitus, which is associated with excessive sweet urine (known as “glycosuria”) but there are several rarer conditions also named diabetes. The most common of these is diabetes insipid us in which the urine is not sweet (insipid us meaning “without taste” in Latin); it can be caused either by kidney or pituitary gland.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1555
1.2 Classification-
Many types of diabetes are recognized. The principal three are-
Diabetes mellitus type 1 results from the body’s failure to produce insulin. It is a disease caused by the lack of insulin. Insulin must be used in Type I, which must be injected.
Diabetes mellitus type 2 results from Insulin resistance, a condition in which cells fail to use insulin properly, sometimes combined with relative insulin deficiency.
THE WAY our bodies use digested food for growth and energy. Most of the food we eat is broken down by the digestive juices into a simple sugar called glucose. Glucose is the main source of fuel for the body.
After digestion, the glucose passes into our bloodstream where it is available for body cells to use for growth and energy. For the glucose to get in the cells, insulin must be present. Insulin is a hormone produced by the pancreas, a large gland behind the stomach.
When we eat, the pancreas is supposed to automatically produce the right amount of Insulin to move the glucose from our blood into our cells. If your body does not make enough insulin or the insulin does not work right, the sugar cannot get into the cells. It stays in the blood. This makes your blood sugar level high, caushing you to have diabetes. As a result, glucose builds up in the blood, overflows into the urine, and passes out of the body. Thus, the body loses its main source of fuel even though the blood contains large amounts of giucose. (Noninsulin dependent diabetes). /Diabetes 2.
[adsense:468x15:2204050025]
Treatments include
(1) Agents that increase the amount of insulin secreted by the pancreas,
(2) Agents that increase the sensitivity of target organs to insulin, and
(3) Agents that decrease the rate at which glucose is absorbed from the gastrointestinal
The goal of treatments for diabetes mellitus is glycemic control that is the prevention of hyperglycemia. Chronic hyperglycemia leads to abnormal glycoslyation of proteins, as well as other chemical changes. Ultimately, the various chemical changes due to hyperglycemia are toxic to certain tissues and diabetic complications ensue. The major diabetic complications are listed below.
Cardiovascular Disease (heart attack, stroke, peripheral vascular disease)
Nephropath(kidneyfailure)
Retinopathy(blindness)
Peripheral Neuropathy (loss of sensation, autonomic dysfunction)
Foot Ulcers (amputation)
Hyperglycemia causes the non-enzymatic glycosylation of proteins, below are listed major drug treatments for diabetes mellitus
1.3 Drugs to control hyperglycemia
Anti-diabetic medications treat diabetes mellitus by lowering glucose levels in the blood
1. Insulin
2. 2. Secretagogues
2.1 Sulfonylureas
2.1 Nonsulfonylurea secretagogues
3. Peptide analogs=Incretin-Based Therapies
4. Alpha-glucosidase inhibitor
5. Sensitizers
- 5.1 Biguanides
- 5.2 Thiazolidinediones
1. Insulin
Insulin therapy is necessary for type 1 diabetics because they have an absolute insulin deficiency due to the autoimmune destruction of the pancreatic beta cells
2.Secretagogues: Drugs that Increase Insulin Secretion
2.1 Secretagogues: Sulfonylureas
2.2 Nonsulfonylurea secretagogues Meglitinides
These drugs bind to and block the ATP-sensitive K+ channel on pancreatic beta cells, causing depolarization and increased insulin secretion
3. Peptide analogs=Incretin-Based Therapies
Incretinsare gastrointestinal hormones that increase insulin secretionSitagliptin, saxagliptin, and linagliptin are DPP-4 inhibitors; prolong the action of native incretins
4. Alpha-glucosidase inhibitors: Drugs that Decrease Glucagon Secretion
Reducing inappropriate glucagon secretion and hepatic glucose production helps to limit hyperglycemia. On type of drug in this category would be the GLP-1 agonists such as exenatide and liraglutide.
5. Insulin Sensitizers
Metformin (Biguanides)works by indirectly activating AMP-activated kinase (AMPK), a protein that works as a cellular energy sensor and regulator of metabolism
Thiazolidinediones(TZD’s; pioglitazone, rosiglitazone) are agonists for an intracellular receptor known as PPAR-gamma. This receptor is primarily expressed in adipocytes. Thiazolidinediones affect adipocyte gene expression, ultimately causing decreases in circulating fatty acids. They also affect adipocyte secretion of regulatory molecules: adipocytes secrete more adiponectin, which increases insulin sensitivity, and fewer adipokines that cause insulin resistance.
Two meta-analysis studies published in 2007 have linked use of rosiglitazone (trade name: Avandia) to an increased risk of heart attack. The FDA now requires a warning on the label for Avandia, and has recently (September 2010) made recommendations against prescribing it for new patients except under special circumstances.
2. PPARs
2.1 HISTORY- PPARs were originally identified in Xenopus frogs as receptors that induce the proliferation of peroxisomes in cells.
The first PPAR (PPARα) was discovered during search of agents then referred to as peroxisome proliferators,
After PPARδ (delta) was identified in humans in 1992, it closely related to the PPARβ (beta) previously described during the same year in other animals (Xenopus).
2.2 DEFINATION-PPAR: Abbreviation for peroxisome proliferator-activated receptor.
The peroxisome proliferator-activated receptors(PPARs) are a group or member of nuclear receptor proteins that function as transcription factors regulating the expression of genes.
Among the NHR superfamily are receptors for steroid, thyroid, and retinoid hormones, which function as nuclear trans-acting transcriptional regulatory factors.
2.3 STRUCTURE-
Like other nuclear receptors, PPARs are modular in structure and contain the following functional domains:
- (A/B) N-terminal region
- (C) DBD (DNA-binding domain)
- (D) flexible hinge regio
- (E) LBD (ligand binding domain)
- (F) C-terminal region
The amino terminal (A/B) domain contains a ligand indepent activation function 1 (AF -1) that contains a mitogen activated protein kinase phosphorylation site.
The DBD contains two zinc finger motifs, which bind to specific sequences of DNA known as hormone response elements when the receptor is activated. The DNA binding domain © is highly conserved and its zinc finger domain is a common attribute amongst all members of the NHR family.
The DNA binding domain is linked to the C terminal ligand –binding domain (E/F) by a ‘hinge’ region.the E/F domains function to dimerize PPARs with RXRs and provide the ligand dependent transactivation function of the receptor. The D domain is a docking domain for cofactors.
2.4 PPAR –DNA binding property
Peroxisome proliferator-activated receptor (PPAR) form heterodimers with retinoid X receptors (RXRs) and these heterodimers regulate transcription of various genes.
All PPARs hetrodimerize with the RXR andbindsto specific regions on the DNA of target genes.PPARs stimulate gene expression by binding to conserved sequences of DNA known as peroxisome-proliferator resonse elements (PPREs), which are present in the promoter of their target genes.ligand activation and binding of the PPARγ/RXR heterodimer are accompanied by the recruitment of co-activators. Without ligands, the heterodimers are physically bound with co-repressor complexes that suppress gene transcription. Upon binding of the ligand to its recap[tors, the nuclear receptor co-repressor(N-CoR)- Containing co-repressor complexes are replaced with co-activator complexes.these co-activator complexes are then linked to the basal transcription apparatus, initiating transcription
The PPAR: RXR heterodimer exists in both an active and inactive state. When inactive, it is bound to corepressors such as the NCOR (Nuclear Receptor Corepressor) or the SMRT (Silencing Mediator for Retinoid and Thyroid Hormone Receptor). In the presence of ligand for either PPAR or RXR, the corepressors dissociate so that the ligand can bind and activate co-activators.
When the PPAR: RXR complex is activated, it binds to PPRE in the 5'region of target genes to induce transcription.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
FUNCTION-
PPARs are involved in several aspects of tissue differentiation and development specifically in adipose tissue, brain, placenta and skin. The developmental expression of PPARs is not known in human.
They play essential role in regulation of diverse biological processes like-
1. Cellular differentiation and development
2. Metabolism homeostasis/regulate bodymetabolism (carbohydrate, lipid, protein)
3. Pattern formation
4. Lipid sensor
5. Another important aspect of PPARs action is repression of inflammatory pathway by transrepression of transcription factors or it may regulate oxidative pathway.
The function of PPARs is modified by the precise shape of their ligand –binding domain, and by a number of coactivator and corepressor proteins.these coactivators and/or repressors in conjugation with with retinoid x receptors (RXR) can stimulate or inhibit PPAR function.
3. TYPES OF PPARS-
Despite their structural similarities, each member of the PPAR family is localized to certain parts of the body. Location of receptor partially determines their function in the body and also the different roles they can play in medicine as drug targets.
Three isoforms of PPAR have been identified ; α(alpha) ; β/δ(beta/delta) ;γ(gamma) also known as NRIC1, NR1C2, and NR1C3, respectively. The PPARs though structurally homologous have different tissue distribution. They have distinct but overlapping biological functions depending on their target genes and tissue distribution.
* α (alpha) - expressed in liver, kidney, heart, muscle, adipose tissue, and others[5] like brown fat,heart,diaphragm,oesophage,immune system, intestine.
• β/δ (beta/delta) - expressed in many tissues but markedly in brain, adipose tissue, and skin .
• γ (gamma) –expressed in adipose tissue,colon,cecum,immune system, cardiovascular system although transcribed by the same gene, this PPAR through alternative splicing is expressed in three forms:
- γ1 - expressed in virtually all tissues, including heart, muscle, colon, kidney, pancreas, and spleen
- γ2 - expressed mainly in adipose tissue (30 amino acids longer)
- γ3 - expressed in macrophages, large intestine, white adipose tissue.
Table 1: Human PPARs as Targets in the Metabolic Syndrome
|
Receptor |
Functions |
Conditions targeted by Agonists |
|
PPARα |
Controls lipid metabolism. |
*Atherogenic dyslipidemia (Elevated serum Triglycerides and low HDL). *Target of fibrate drugs. |
|
PPARδ |
Appears to control multiple aspects of the metabolic syndrome. |
Metabolic syndrome, Obesity, atherosclerosis |
|
PPARγ |
Controls glucose metabolism and adipocyte differentiation. |
*Insulin resistance in type 2 Diabetes. *Target of thiazolidinediones. |
Besides this, PPAR γperforms-
1. Controls glucose metabolism and adipocyte differentiation.
2. PPAR gamma promotes adipocyte differentiation to enhance blood glucose uptake initiation.
3. It regulates the development of adipocytes (fat cells). Adipocyte differentiation, metabolism of lipids, fatty acid storage and glucose metabolism and reduces insulin resistance.
4 PPAR-gamma is made primarily in fat cells and affects the production of fat
5. The genes activated by PPARG stimulate lipid uptake and adipogenesis by fat cells.
6. PPAR-gamma has been implicated not only diabetes 2 but in the pathology of numerous diseases including- obesity, diabetes, atherosclerosis, and cancer and decreases the inflammatory response.
4. PPAR SINGLING PATHWAY AND ITS AGONIST
PPAR signaling pathway - Homo sapiens (humans)
Peroxisome proliferator-activated receptors (PPARs) are activated by fatty acids and their derivatives.
(PPARalpha, beta/delta, and gamma) showing different expression patterns in vertebrates. Each of them is encoded in a separate gene and binds fatty acids and eicosanoids.
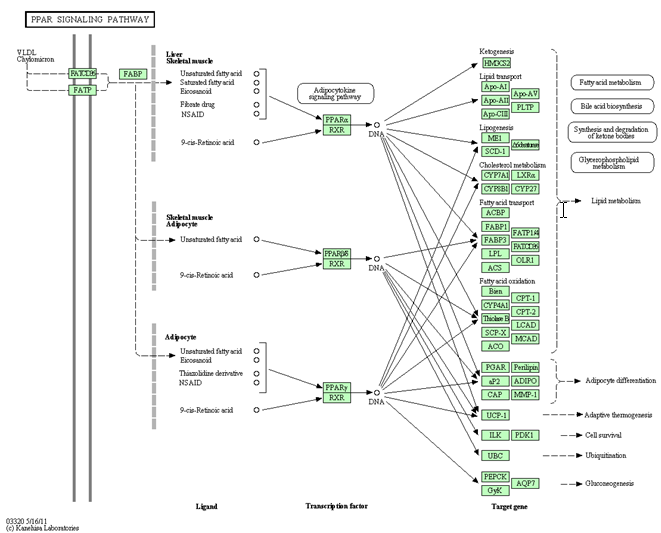
PPAR agonists
A family of drugs that activate certain proteins in the body called peroxisome proliferator-activated receptors (PPARs). PPARs regulate the expression of genes that affect blood lipid metabolism, the generation of adipocytes (fat cells), and blood glucose control.
The PPAR agonists help to improve blood glucose levels and levels of blood lipids (fats and cholesterol) and may also reduce risks of atherosclerosis
PPAR Agonists-
1. PPARgamma agonist-insulin sensitizers for type 2 diabetes
–– Approved drugs – Actos (pioglitazone), Avandia (rosiglitazone)
2. PPAR alpha agonists for dyslipidemia increase HDL; decrease TG, no effects on LDL. Approved drugs – fibrates
3. PPAR dual agonist (alpha, gamma) being developed for combined treatment of type 2 diabetes and dyslipidemia Muraglitazar, Tesaglitazar, Ragaglitazar, Naveglitazar
4. PPAR delta agonists–– being developed for obesity
5. PPAR pan agonists (alpha/delta/gamma) – being developed for type diabetes and dyslipidemia.
5. PPARγ as TARGET FOR TYPE-2 DIABETES-
Many insulin sensitizing drugs used in the treatment of diabetes target PPARG as a means to lower serum glucose without increasing pancreatic insulin secretion
Type 2 diabetes mellitus is the most frequent endocrine-metabolic diseases and are characterized by insulin resistance and insulin secretion defects that can be demonstrated through several alterations in carbohydrates, lipids, and protein metabolism. The peroxisome proliferator-activated receptors have been identified as key regulators of glucose and lipid metabolism, because they act as transcription factors
This receptor is primarily expressed in adipocytes. Adipocyte helps in the regulation of energy homeostasis, storing energy in times of nutritional excess and releasing that energy when needed in times of nutritional deprivation. Adipocytes secrete more adiponectin, which increases insulin sensitivity, and fewer adipokines that cause insulin resistance. PPARγ binds a variety of natural ligands including fatty acids, eicosanoides, and other natural lipid ligands. It predominantly stimulates adipocyte differentiation and regulates lipid metabolism in mature adipocytes..PPARγ is expressed at extremely high levels in adipose tissue, macrophages, and the large intestine, and controls lipid adipogenesis and energy conversion.
PPARγ is responsible for lipid metabolism and cellular energy homeostasis. It binds genes that transcribe proteins which act as fatty acid transporters, are critical in insulin signaling and glucose transport, catalyze glycerol synthesis from triglycerides, and catabolize lipids. This makes PPARγ an ideal target to treat Diabetes.
PPARG Controls glucose metabolism and adipocyte differentiation to enhance blood glucose uptake initiation. The genes activated by PPARG stimulate lipid uptake and adipogenesis by fat cells PPAR-gamma is made primarily in fat cells and affects the production of fat.
It regulates the development of adipocytes (fat cells). Adipocyte differentiation; metabolism of lipids; fatty acid storage and glucose metabolism and reduces insulin resistance
PPAR-gamma has been implicated not only diabetes 2 but in the pathology of numerous diseases including-obesity, diabetes, atherosclerosis, as cancer and decreases the inflammateory response.
Insulin Sensitizers drugs are used to counteract the key problem of type 2 diabetes against insulin resistance (the inability of tissues such as muscle and fat to utilize insulin efficiently for the uptake of glucose GLUT4, the protein primarily responsible for transporting glucose into muscle and fat cells in response to insulin.
5.1 Peroxisome proliferator-activated receptor gamma
Peroxisome proliferator-activated receptor gamma(PPAR-γ or PPARG), also known as the glitazone receptor, or NR1C3 (nuclear receptor subfamily 1, group C, member 3) is a type II nuclear receptor that in humans is encoded by the PPARG gene
The gene for PPAR-gamma is in chromosome band 3p25
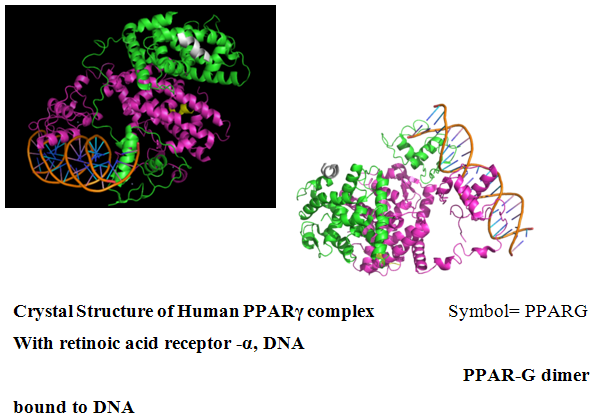
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
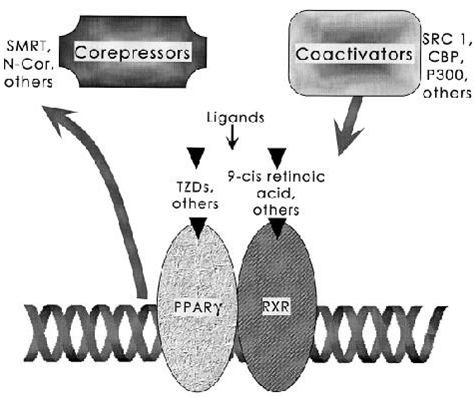
FIG. 3. Docking of cofactors for PPA R - through ligand binding. The PPA R - /RXR heterodimer binds to distinct DNA sequence elements called DR-1 sites. The binding of ligands to nuclear receptors is now understood to stimulate release of negative factors (corepressors) and trigger binding of positive cofactors (coactivators). Many of these proteins modulate the acetylation state of histones and serve to open chromatin for more efficient transcription.
In diabetes mellitus type 2 thiazolidinediones is the only approved drug.Thiazolidinediones (TZD’s; pioglitazone, rosiglitazone) are agonists for an intracellular receptor known as PPAR-gamma
5.2 the Regulation of PPARγ protein activity
PPARγ is a major regulator of adipogenesis via stimulation by fatty acids, and under circumstances of plenty substrate access the PPARγ activity is increased, whereas under circumstances of substrate depletion, for example, fasting, exercise or perhaps growth, the expression and/or activity of PPARγ seems to be attenuated.
6. PPARγ AGONIST
6.1Thiazolidinedione (TZD)-
The only approved use of the thiazolidinediones is in diabetes mellitus type 2.The thiazolidinediones also known as glitazones, are a class of medications used in the treatment of diabetes mellitus type 2. They were introduced in the late 1990s.
They are a new class of orally active drugs that are designed to enhance the actions of insulin. These agents reduce insulin resistance by increasing insulin-dependent glucose disposal and reducing hepatic glucose output.
Clinical studies in patients with type II diabetes, as well as other syndromes characterized by insulin resistance, have demonstrated that thiazolidinediones may represent a safe and effective new treatment.
This regulation of gene expression appears to be mediated by the interactions of thiazolidinediones with a family of nuclear receptors known as the peroxisome proliferator-activated receptors (PPARs).In subsequent binding studies, TZDs were demonstrated to enhance insulin action by activating peroxisome proliferator-activated receptor gamma (PPARgamma). PPARgamma is a member of the ligand-activated nuclear receptor superfamily that promotes adipogenesis and enhances insulin sensitivity by controlling the expression of genes in glucose and lipid metabolism.
The TZD (thiazolidenediones) are compounds that acts as agonist of the peroxisome proliferator activated receptor gamma increasing the tissue sensibility (muscle,adiposity tissue and liver) to the insulin action. The only approved use of the thiazolidinediones is in diabetes mellitus type 2
6.1.1 Reason for considering TZD as ligand for PPARG
Pioglitazone increased transcriptional activity from theaP2 enhancer and apparently did, so through the differentiation-linked DNA site (ARE6) when we cloned and identified the ARE6 binding factor as the PPA R -/RXR heterodimer, two groups independently asked whether the TZD drugs were acting as direct agonists for P PA R - Gamma. These studies identified BRL49653 and pioglitazoneas direct ligands of PPAR- with positive activity gene transcription importantly, the TZDs were also shown to be highly selective for PPAR- , as they had very minimal activity toward PPAR-alpha or PPARdelta- . TZDs had been shown to stimulate adipogenesis in preadipocyte cell lines, though the mechanism of action of the drugs in these early studies was not known.
The evidence that PPA R - is the major receptor mediating the antidiabetic activity of the TZDs is now very strong, based on the following multiple lines of pharmacological evidence.
1. Each of the TZD drugs binds to and activates PPA R - in the same concentration range that has antidiabetic activity
2. Among many TZDs surveyed, the rank order of potency of their antidiabetic activities closely matches the rank order of their affinities for PPA R .
3. Potent and selective ligands for PPA R - outside of the TZD class has now been developed on the basis of their activation of PPAR. These have antidiabetic actions in preclinical models of insulin resistance and diabetes
4. Ligand stimulation of RXR, the heterodimeric partner of PPAR , also improves insulin sensitivity in vivo.
5. No other receptor for the TZD drugs taken together, these data makes a compelling case that PPAR is the major functioning receptor for the common TZD actions in diabetes. As been identified Thiazolidinediones affect both adipocyte gene expressions, ultimately causing decreases in circulating fatty acids and adipocyte secretion of regulatory molecules:
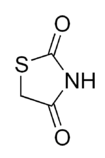
6.1.2 The chemical structure of thiazolidinedione
6.1.3 Mechanism of action
Thiazolidinediones or TZDs act by activating PPARs (peroxisome proliferator-activated receptors), a group of receptor molecules inside the cell nucleus, specifically PPARγ (gamma). The ligands for these receptors are free fatty acids (FFAs) and eicosanoids. When activated, the receptor migrates to the DNA, activating transcription of a number of specific genes.
By activating PPARγ
* Insulin resistance is decrease
*Adipocyte differentiation is modified [1]
- VEGF-induced angiogenesis is inhibited[2]
- Leptin levels decrease (leading to an increased appetite) lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-d
- Levels of certain interleukins (e.g. IL-6) fall
- Adiponectin levels rise
- TZDs also increase the synthesis of certain proteins involved in fat and glucose metabolism, which reduces levels of certain types of lipids, and circulating free fatty acids. TZDs generally decrease triglycerides and increase high-density C).. Stroke.[3]
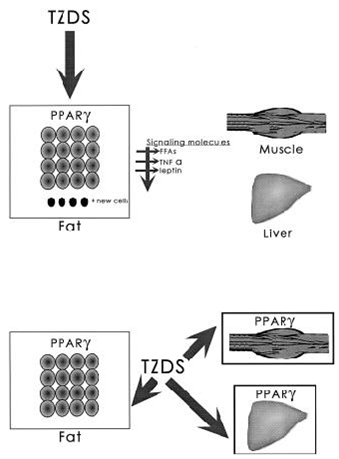
6.1.4 FIG. 2. Tissue targets for TZD drugs
In A, the primary tissue target for the TZDs is PPA R – in fat. Effects on insulin action in other tissues would then occur as a consequence of alterations in signaling molecules produced by fat, such as free fatty acids, TNF- , leptin, or others.
Alternatively (B), TZDs may have direct actions on PPA R - in fat, muscle, and liver and control the expression of genes that influence insulin action in these tissues.
6.1.5 Insulin sensitization effects by TZD-
*TZD-direct effect on fat, muscle, liver
*Effects on fat cell differentiation
*Control of adipose cell singling
Adipose cell sends molecular signals to other tissues that are participating in systemic insulin sensitivity i.e.TNFα energy metabolism. PPAR gamma activation control gene that regulate andleptin.
During chronic overfeeding and adipocyte hypertrophy, the activity of the PPARγ receptor is attenuated, and hence the adipocytes have a lowered fat-depositioning capacity, giving rise to increased plasma levels of FFAs, which subsequently may lead to insulin resistance in adipose tissue, liver, and muscle. The TZDs are a group of PPARγ receptor agonists which in adipose tissue strongly stimulate PPARγ -mediated transcription of the genes encoding proteins involved in adipose tissue lipid metabolism, and these drugs are probably able to recruit and convert noncommitted preadipocytes into phenotypical adipocytes.
6.2Members of the class
Chemically, the members of this class are derivatives of the parent compound thiazolidinedione, and include:
- Rosiglitazone (Avandia), which was put under selling restrictions in the US and withdrawn from the market in Europe due to an increased risk of cardiovascular events.
- Pioglitazone (Actos), France and Germany have suspended the sale of the diabetes drug Actos after a study suggested the drug, also known as pioglitazone, could raise the risk of bladder cancer.[4]
- Troglitazone (Rezulin), which was withdrawn from the market due to an increased incidence of drug-induced hepatitis.
* New PPARgamma agonists: benzimidazole derivatives
Experimental agents include netoglitazone, an antidiabetic agent, rivoglitazone, and the early non-marketed thiazolidinedione ciglitazone.
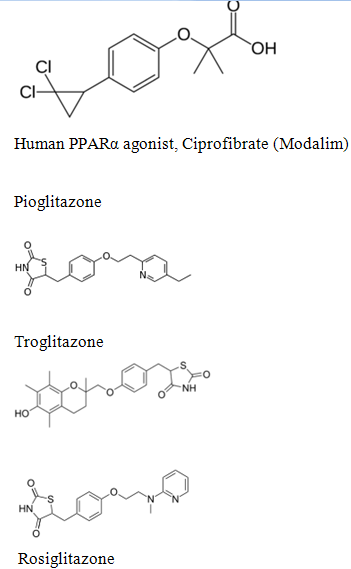
Natural ligands/Agonist.-
*The first natural ligand described was an unusual prostanoid15- d e o x y 1 2, 1 4PG J2
*More recently, several polyunsaturated fatty acids, such as linoleic acid, have also been found to bind directly to PPA R –Gamma
- Dietary polyunsaturated fatty acid eicosapentanoic acid
- Oxidized low-density lipoprotein particles (oxLDL) 9-HODE and 13-HODE were identified as endogenous ligands.
It is significant that all of the identified natural ligands are lipophilic carboxylic acids.
PPAR gamma binds polyunsaturated fatty acids like linoleic acid, linolenic acid, and eicosapentaenoic acid at affinities that are in line with serum levels found in the blood. PPARα binds a variety of saturated and unsaturated fatty acids including palmitic acid, oleic acid, linoleic acid, and arachidonic acid.[10] PPARδs ligand selectivity is intermediate between that of the other isotypes and is activated by palmitic acid and a number of eicosanoids.[11]
Artificial PPARγ ligands/Agonist
The discovery that PPARγ is the molecular target of theTZDs, for example, Rosiglitazone, provided the critical insight necessary for the rational design of new antidiabetic drugs. Hence, a series of agonists optimized against the PPARγ receptor have been developed. These include GI262570 (Figure 1), GW1929, and GW7845. Temporary studies have shown that these compounds activate PPARγ at nanomolar concentrations, and that they in vivo reduce plasma glucose and triglyceride levels, accompanied with a rise in HDL cholesterol.20 Recently, the novel PPARγ antagonists GW0072,22 bisphenol A diglycidyl ether (BADGE),23 PD06823524,25 and LG100641,24,25 have been identified, and they are all able to antagonize PPARγ agonist-induced adipogenesis.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
7. BENEFICIAL EFFECTS OF PPARγ AGONIST
The TZDs are a relatively new class of oral antidiabetic drugs, and they are often referred to as 'insulin sensitizers'.
- The first of this type of compounds was Ciglitazone, which was synthesized in 1982, and subsequently Pioglitazone, Englitazone, Troglitazone, Rosiglitazone, and Darglitazone were synthesized
- Only Troglitazone, Pioglitazone, and Rosiglitazone were evaluated in clinical studies,
- Troglitazone was approved for clinical use by the US Federal Drug Administration (FDA) in 1997, but were subsequently withdrawn from the market in March 2000 because of idiosyncratic liver toxicity.
- Rosiglitazone ('Avandia' from GlaxoSmithKline) and Pioglitazone ('Actos' from Eli Lilly) were approved by FDA in 1999, and at the moment approximately three million type II diabetics are being treated by these two drugs in the US.
- Pioglitazone treatment, in contrast, has shown significant protection from both micro- and macro-vascular cardiovascular events and plaque progression.
These three PPARγ agonists display distinct characteristics since, although they all contain the same active TZD ring, they have different side chains. As a consequence, these PPARγ agonists differ in their pharmacological potency;
- The PPARγ-binding affinity of Rosiglitazone is 100-fold greater than that of Troglitazone and over 30 times that of Pioglitazone. Despite their different degrees of potency, all the PPARγ agonists are shown to be effective in relieving insulin resistance
- The beneficial metabolic effects of PPARγ agonist treatment of human type II diabetics include83
1. Reduction in postprandial glucose, fasting plasma glucose and HbA1c,
2. Increased insulin sensitivity and improved pancreatic island ß-cell function;
3. Increase in HDL levels and (variable) lowering of LDL levels;
4. Lowering of diastolic blood pressure, decreased microalbuminuria, and increased levels of the fibrinolytic substances plasminogen activator inhibitor 1 (PAI-1) and tissue plasminogen activator (tPA).
5. By enhancing insulin sensitivity and thereby reducing the burden on the pancreas, the PPARγ agonists may also have the potential to improve ß-cell function and thereby delay disease progression. The powerful combination of improvement in ß-cell function and reduction in insulin resistance is key to the success of controlling type II diabetes.
6. PPAR-gamma agonists have been used in the treatment of dyslipidaemia and hyperglycemia.
7. Recently pioglitazone, a PPARg agonist has been shown to be effective in reducing inflammation in Parkinson's disease models. Levels of MMPs and microglia (and therefore TNFa and other cytokine levels) were found to be reduced. Thus it has been shown to be neuroprotective in MPTP mouse models
Peroxisome proliferator-activated receptor-gamma agonist improves skeletal muscle insulin signaling in the pregestational intrauterine growth-restricted rat offspring.
These drugs produce several of adverse effects such as – weight gain, edema, anemia, pulmonary edema and congestive cardiac failure. Even their use have been related for some studies to an increased in the myocardium, infarct risk; this co-relation has not been a strong determinant to remove them from markets.
8. FUTURE DEVELOPMENT
8.1 1st Approach
*Partial PPAR agonists have the potential to retain the desired efficacy and beneficial effects of full PPAR agonists while diminishing their unwanted effects. Especially in the case of PPARγ agonists, companies are developing partial agonists, with the goal of retaining the beneficial effects of this class of agents, while diminishingtheir adverse effects. Companies that are developing such agents include Metabolex, Roche, Evolva, and Astellas.
* Metabolex’s lead candidate metaglidasen has a different chemical structure and mechanism of action than the insulin sensitizers. Metaglidasen is not a TZD.
*Metaglidasen is apartial agonist of the PPAR-γ receptor and selectively modifies gene expression needed for insulin sensitization without activating the genes responsible for weight gain and edema.
*10, 2006, Metabolex announced that it had initiated a Phase II/III clinical trial Of metaglidasen in patients with type 2 diabetes. In a previous Phase II clinical trial, metaglidasen was well tolerated and lowered blood glucose and triglycerides.
* Two angiotensin II receptor blockers
(ARBs) approved for the treatment of hypertension, telmisartan and irbesartan are also partial agonists of PPARγ. Both of these agents have established safety profiles.
Studies with partial agonists of PPARγ suggest that a focus on partial PPAR agonistsmay be a way of developing agents that have the desired efficacy of PPAR agonists without at least some of their potential adverse effects. However, development of PPARagonist remains a challenging endeavor.
8.2 2nd Approach
DEVELOPMENT OF BETTER PPA R - L I G A N D S–
The TZDs were originally developed without any knowledge of their molecular targets; there is hope that the research described above will lead to the development of new and better PPA R -γ ligand.s
* The word “better” can be thought of in at least two ways: potency and efficacy
* Given that the potency of a particular ligand will ultimately depend on its affinity for PPA R - γ, with the use of modern drug-screening procedures, large chemical libraries, and combinatorial chemistry methods, it is very likely that agents with higher affinity for PPA R - can be found
* Improvements in efficacy may reside in two areas: ligands that have more full agonist activity on PPA R - or agents that stimulate the PPA R - /RXR heterodimer.
Recognizing the predominantly beneficial effects of activating the PPARs, scientists have set about to design drugs that activate both PPAR-alpha and PPAR-gamma, which would not only help sensitize the body to insulin but would also have a beneficial effect on lipids. This novel class of Type 2 diabetes medicines, called dual PPAR activators (or PPAR-alpha/gamma agonists or “glitazars”), include the investigational drugs muraglitazar and tesaglitazar. They have more adverse effecs hence not usually used.
9. CONCLUSION
Conclusion and perspectives
*Type 2 diabetes mellitus is endocrine-metabolic disease, characterized by insulin resistance and insulin secretion defects the peroxisome proliferator-activated receptors work as key regulators of glucose and lipid metabolism, and cellular energy homeostasis. It binds genes that transcribe proteins which act as fatty acid transporters, are critical in insulin signaling and glucose transport, catalyze glycerol synthesis from triglycerides, and catabolize lipids. This makes PPARγ an ideal target to treat Diabetes
*The TZD act as agonists of the PPARG increasing the tissues sensibility (muscle, adiposity tissue, and liver) to the insulin action; hence used in treatment of type 2 diabetes mellitus
*These drugs produce several of adverse effects. It is the only approved drug in type 2..
10. REFERENCES
1. Flier JS: Peroxisome proliferator-activated receptor gene expression in human tissues: effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest 99:2416–2422, 1997
2. Henry RR: Thiazolidinediones. Endocrinol Metab Clin North Am 26:9553–9573, 1997
3. With peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad
Sci USA 94:4318–4323, 1997
4. Bastie C, Luquet S, Holst D, Jehl-Pietri C, Grimaldi PA. Alterations of peroxisome proliferator-activated receptor delta activity affect fatty acid-controlled adipose differentiation. J Biol Chem 2000; 275: 38768–38773. |
5. Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem 2001; 70: 341–367.
6. Ibrahimi A et al. Evidence for a common mechanism of action for fatty acids and thiazolidinedione antidiabetic agents on gene expression in preadipose cells. Mol Pharmacol 1994; 46: 1070–1076
7. Mukherjee R et al. A selective peroxisome proliferator-activated receptor-gamma (PPAR gamma) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocr 2000; 14: 1425–1433.
8. Morrison RF, Farmer SR. Hormonal signaling and transcriptional control of adipocyte differentiation. J Nutr 2000; 130: 3116S–3121S. |
9. Kubota N et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 1999; 4: 597–650
10. Rosen ED et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999; 4: 611–617.
11. Hotta K et al. Relationships of PPARgamma and PPARgamma2 mRNA levels to obesity, diabetes and hyperinsulinaemia in rhesus monkeys. Int J Obes Relat Metab Disord 1998; 22: 1000–1010.
12. Rieusset J et al. Insulin acutely regulates the expression of the peroxisome proliferator-activated receptor-gamma in human adipocytes. Diabetes 1999; 48: 699–705.
13. Yakubu-Madus FE, Stephens TW, Johnson WT. Lipid lowering explains the insulin sensitivity enhancing effects of a thiazolidinedione, 5-(4-(2-(2-phenyl-4-oxazolyl) ethoxy) benzyl)-2,4 thiazolidinedione. Diabetes Obes Metab 2000; 2: 155–163.
14. Chao L et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidaemic, effect of thiazolidinediones. J Clin Invest 2000; 106: 1221–1228
15. Yamauchi T et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001; 276: 41245–41254
16. Deeb SS et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 1998; 20: 284–287.
17. Schmitz OE, Brock B, Madsbad S, Beck-Nielsen H. [Thiazolidinediones--a new class of oral antidiabetics]. Ugeskr Laeger 2001; 163: 6106–6111.
18. Zinman B. PPAR gamma agonists in type 2 diabetes: how far have we come in 'preventing the inevitable'? A review of the metabolic effects of rosiglitazone. Diabetes Obes Metab 2001; 3(Suppl 1): S34–S43.
19. Kelly IE, Han TS, Walsh K, Lean ME. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 1999; 22: 288–293.
20. Nakamura T et al. Thiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation—double-blind placebo-controlled trial. Diabetes Res Clin Pract 2001; 54: 181–190.
21. Staels B, Fruchart JC (2005). "Therapeutic roles of peroxisome proliferator-activated receptor agonists". Diabetes 54 (8): 2460–70.
22. Fiévet C, Fruchart JC, Staels B (2006). "PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome". Current Opinion in Pharmacology 6 (6): 606–14.
23. Rangwala SM, Lazar MA (2004). "Peroxisome proliferator-activated receptor gamma in diabetes and metabolism". Trends Pharmacol. Sci. 25 (6): 33.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









