About Author: Rinki Verma (Research Fellow)
Center of Experimental Medicine and Surgery,
Institute of Medical sciences,
Banaras Hindu University,
Varanasi, India
Abstract
Genetic instability caused by secondary metabolites produced by fungus. These mycotoxins are chemical compound which are naturally toxic to cells. Because of ability to generating ROS and RNS caused oxidative stress. Due to their toxicity properties implicate apoptosis and form cancer cell. DNA replication are going to impaired and become damage. Mycotoxins are suppressed tumor suppressing gene and convert proto-oncogene in the oncogene. In this review we explain the control exposure of mycotoxins and provides guidelines to farmers because they are directly contact to these compounds.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1099
Introduction
According to WHO humans and animals may be exposed to mycotoxins through ingestion, inhalation or skin contact. Since, not much work has been carried out on skin related effects of Mycotoxin, WHO had highlighted the need for generating toxicological data on mycotoxins through dermal exposure. A mycotoxin (from Greek (mykes, mukos) "fungus" and Latin (toxicum) "poison") is a toxic secondary metabolite produced by an organism of the fungus kingdom, including mushrooms, molds, and yeasts. The term 'mycotoxin' is usually reserved for fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the minute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. One mold species may produce many different mycotoxins and/or the same mycotoxin as another species. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the production of mycotoxins is not yet known; they are neither necessary for growth nor the development of the fungi. The production of toxins is dependant on the surrounding intrinsic and extrinsic environments and the toxins vary greatly in their severity, depending on the organism infected and its susceptibility, metabolism, and defense mechanisms. Some of the health effects found in animals and humans include death, identifiable diseases or health problems, weakened immune systems without specificity to a toxin, and as allergens or irritants.
Some mycotoxins are harmful to other micro-organisms such as other fungi or even bacteria; penicillin is one example. Mycotoxins can appear in the food chain as a result of fungal infection of crops, either by being eaten directly by humans, or by being used as livestock feed. Mycotoxins greatly resist decomposition or being broken down in digestion, so they remain in the food chain in meat and dairy products. Even temperature treatments, such as cooking and freezing, do not destroy mycotoxins.
Food-based mycotoxins were studied extensively worldwide throughout the 20th century. In Europe, statutory levels of a range of mycotoxins permitted in food and animal feed are set by a range of European directives and Commission regulations. The U.S. Food and Drug Administration has regulated and enforced limits on concentrations of mycotoxins in foods and feed industries since 1985. It is through various compliance programs that the FDA monitors these industries to guarantee that mycotoxins are kept at a practical level. These compliance programs sample food products including peanuts and peanut products, tree nuts, corn and corn products, cottonseed, and milk. There is still a lack of sufficient surveillance data on some mycotoxins that occur in the U.S. which is largely due to the lack of reliable analytical methods.
Classification of mycotoxin:
• Aflatoxins are a type of mycotoxin produced by Aspergillus species of fungi, such as A. flavus and A. parasitic (Martin et al., 2001). The umbrella term aflatoxin refers to four different types of mycotoxins produced, which are B1, B2, G1, and G2. Aflatoxins in spices marketed in Portugal". Aflatoxin B1, the most toxic, is a potent carcinogen and has been directly correlated to adverse health effects, such as liver cancer, in many animal species. Aflatoxins are largely associated with commodities producedin the tropics and subtropics, such as cotton, peanuts, spices, maize and pistachios.
• Ochratoxin is a mycotoxin that comes in three secondary metabolite forms, A, B, and C. All are produced by Penicillium and Aspergillus species. The three forms differ in that Ochratoxin B is a non-chlorinated form of Ochratoxin A and that Ochratoxin C is an ethyl ester form Ochatoxin A (Bayman and Baker, 2006). Aspergillus ochraceus is found as a contaminant of a wide range of commodities including beverages such as beer and wine. Aspergillus carbonarius is the main species found on vine fruit, which releases its toxin during the juice making process (Mateo et al., 2007). OTA has been labeled as a carcinogen and a nephrotoxin, and has been linked to tumors in the human urinary track, although research in humans is limited by confounding factors.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
• Citrinin is a toxin that was first isolated from Penicillium citrinum, but has been identified in over a dozen species of Penicillium and several species of Aspergillus. Some of thesee species are used to produce human foodstuffs such as cheese (Penicillium camemberti), sake, miso, and soy sauce (Aspergillus oryzae). Citrinin is associated with yellow rice disease in Japan and acts as a nephrotoxin in all animal species tested. Although it is associated with many human foods (wheat, rice, corn, barley, oats, rye, and food colored with Monascus pigment) its full significance for human health is unknown. Citrinin can also act synergistically with Ochratoxin A to depress RNA synthesis in murine kidneys (Bennett JW and Klich M, 2003).
• Ergot Alkaloids are compounds produced as a toxic mixture of alkaloids in the sclerotia of species of Claviceps, which are common pathogens of various grass species. The ingestion of ergot sclerotia from infected cereals, commonly in the form of bread produced from contaminated flour, cause ergotism the human disease historically known as St. Anthony’s fire. There are two forms of ergotism gangrenous affecting blood supply to extremities and convulsive which affects the central nervous system. Modern methods of grain cleaning have significantly reduced ergotism as a human disease; however it is still an important veterinarian problem. Ergot alkaloids have been used pharmaceutically (Bennett JW and Klich M, 2003).
• Patulin is a toxin produced by the P. expansum, Aspergillus, Penicillium, and Paecilomyces fungal species. P. expansum is especially associated with a range of moldy fruits and vegetables, in particular rotting apples and figs. It is destroyed by the fermentation process and so is not found in apple beverages, such as cider. Although patulin has not been shown to be carcinogenic, it has been reported to damage the immune system in animals (Moss, 2008). In 2004, the European Community set limits to the concentrations of patulin in food products. They currently stand at 50 μg/kg in all fruit juice concentrations, at 25 μg/kg in solid apple products used for direct consumption, and at 10 μg/kg for children's apple products, including apple juice (Moss, 2008).
• Fusarium toxins are produced by over 50 species of Fusarium and have a history of infecting the grain of developing cereals such as wheat and maize (Cornely, 2008). They include a range of mycotoxins, such as: the fumonisins, which affect the nervous systems of horses and may cause cancer in rodents; the trichothecenes, which are most strongly associated with chronic and fatal toxic effects in animals and humans; and zearalenone, which is not correlated to any fatal toxic effects in animals or humans. Some of the other major types of Fusarium toxins include: beauvercin and enniatins, butenolide, equisetin, and fusarins (Desjardins and Proctor, 2007).
• Sterigmatocystin is carcinogenic mycotoxin produced by Aspergillus versicolor and other fungi.
Table-1: Common members of the mycotoxin family
|
Mycotoxin
|
Organism
|
Foods
|
Structure |
|
Aflatoxin
|
A. flavus
|
Corn,peanuts,cottonseed,etc. |
|
|
Ochratoxin A
|
A. ochraceus
|
Corn,barley,wheat, peanuts.
|
|
|
Patulin
|
A. clavatus
|
Silage, applesetc.
|
|
|
Deoxynivalenol |
Fusarium |
Wheat ,grain etc |
|
|
Trichothecenes
|
Fusarium, Myrothecium, Phomopsis
|
Weathered grain, sorghum, pecan pickoutsetc.
|
|
|
Zaralenonee |
Fusarium roseum
|
Corn, sorghum, wheatetc.
|
|
Fig-1. Showing different feasible route of revelation of mycotoxins.

Reactive oxygen species (ROS) are ions or very small molecules that include oxygen ions, free radicals, and peroxides, both inorganic and organic. They are highly reactive due to the presence of unpaired valence shell electrons. ROS form as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling. However, during times of environmental stress (such as for example, UV or heat exposure) ROS levels can increase dramatically, which can result in significant damage to cell structures. This cumulates into a situation known as oxidative stress. They are also generated by exogenous sources such as ionizing radiation.
ROS are generated by different mechanisms. They are constantly produced within the body from normal oxidative metabolism. Sources of ROS are mainly the mitochondrial electron transport chain, but also the arachidonic acid cascade or nitric oxide (Miquel, 1992). During evolution, living organisms have been faced with the necessity to inactivate these free radicals, and they have developed several ways to protect themselves from oxidative attacks. These defense mechanisms include a variety of antioxidant enzymes like superoxide dismutase or glutathione reductase, which catalyzes the NADPH-dependent reduction of glutathione disulfide to glutathione. This reaction is essential for the maintenance of glutathione levels. Glutathione itself plays a major role as a reductant inoxidant-reduction processes, and also serves in detoxification (Carlberg and Mannervik, 1985).
Reactive nitrogen species (RNS) such as nitric oxide (NO) is a ubiquitous messenger molecule in the cardiovascular, nervous and immune systems, and peroxynitrite (ONOO−) as a species inducing nitrative stress. L-arginine derived NO has been found in a wide variety of organisms ranging from mammals to invertebrates, bacteria, and plants. NO is biosynthesized by three distinct mammalian NO synthase isoforms. Neuronal NOS (nNOS or NOS I) and endothelial NOS (eNOS or NOS III) are constitutively expressed in neuronal and endothelial cells, respectivel, and thus, both enzymes are also referred to as cNOS, while inducible NOS (iNOS or NOS II) is expressed in cells involved in inflammation such as macrophages and microglias through stimulation with cytokines and/or endotoxins.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Oxidative stress is caused by an imbalance between the production of reactive oxygen and a biological system's ability to readily detoxify the reactive intermediates within their cells. This reducing environment is preserved by enzymes that maintain the reduced state through a constant input of metabolic energy. Disturbances in this normal redox state can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA.
In chemical terms, oxidative stress is a large rise (becoming less negative) in the cellular reduction potential, or a large decrease in the reducing capacity of the cellular redox couples, such as glutathione (Schafer and Buettner GR, 2001). The effects of oxidative stress depend upon the size of these changes, with a cell being able to overcome small perturbations and regain its original state. However, more severe oxidative stress can cause cell death and even moderate oxidation can trigger apoptosis, while more intense stresses may cause necrosis (Lennon. et al., 1991).
Table - 2: Type of oxidant
|
Oxidant |
Description |
|
•O2-, superoxideanion |
One-electron reduction state of O2, formed in many autoxidation reactions and by the electron transport chain. Rather unreactive but can release Fe2+ from iron-sulfur proteins and ferritin. Undergoes dismutation to form H2O2 spontaneously or by enzymatic catalysis and is a precursor for metal-catalyzed •OH formation. |
|
H2O2, hydrogen peroxide |
Two-electron reduction state, formed by dismutation of •O2- or by direct reduction of O2. Lipid soluble and thus able to diffuse across membranes. |
|
•OH, hydroxyl radical |
Three-electron reduction state, formed by Fenton reaction and decomposition of peroxynitrite. Extremely reactive, will attack most cellular components |
|
ROOH, organic hydroperoxide |
Formed by radical reactions with cellular components such as lipids and nucleobases. |
|
RO•, alkoxy and ROO•, peroxy radicals |
Oxygen centered organic radicals. Lipid forms participate in lipid peroxidation reactions. Produced in the presence of oxygen by radical addition to double bonds or hydrogen abstraction. |
|
HOCl, hypochlorous acid |
Formed from H2O2 by myelo peroxidase. Lipid soluble and highly reactive. Will readily oxidize protein constituents, including thiol groups, amino groups and methionine. |
Role of Reactive Oxygen and Nitrogen Species (ROS/RNS):
a) Toxicity: Several types of compound exert their cytotoxicity by generating reactive oxygen species, notably the superoxide anion radical. These include quinoid and nitroaromatic compounds serving as redox cyclers, i.e. producing superoxide at the expense of NADPH and oxygen catalyzed by cellular reductases. In specialized cell-types employed in defense such as granulocytes, eosinophils and macrophages, myeloperoxidase, NADPH oxidase and nitric oxide synthase have been identified as major sources of reactive oxygen species in cell toxicity (Sies H and de Groot H, 1992).
And exposure to aniline causes selective toxicity to the spleen leading to vascular congestion, hyperplasia, fibrosis and a variety of sarcomas in rats (Bus and Popp, 1987; Khan. et al., 1995, 1997a, 1999a, 2003a and , 2003b). Mechanism (s) by which aniline induces selective toxicity to the spleen is not well understood.
b) Genotoxicity: ROS\RNS can also damage mitochondrial DNA, and such damage has been suggested to be important in several human diseases and in the aging process (Harman. D.1992 and Shigenaga. et al., 1994). ROS/RNS can cause DNA base changes, strand breaks, damage to tumour-suppressor genes and enhanced expression of proto-oncogenes (Cerutti. P. and Jackson. et al., 1994) . And oxidative stress has been shown to induce malignant transformation of cells in culture (Weitzman.et al., 1990 and Zimmerman. et al., 1984 ). However, the development of human cancer depends on many other factors, including the extent of DNA damage (excessive DNA damage can cause cell suicide by activation of PARP; antioxidant defences, DNA repair systems, the efficiency of removal of oxidized nucleosides (e.g. oxo-dGTP) before they are incorporated into DNA, and the cytotoxic effects of ROS in large amounts (a dead cell will not lead to cancer) as well as their growth-promoting effects in small amounts (Burdon, et al., 1995 and Craven. et al., 1986).
c) Carcinogenicity: It is increasingly proposed that reactive oxygen species and reactive nitrogen species (RNS) play a key role in human cancer development (Totter and J. R, 1980). (Routledge. et al. ,1994). Reactive oxygen and nitrogen species are important in mutagenesis and carcinogenesis (Wiseman H, 1996 and Imlay JA, 1988). Enhanced oxidative stress might be associated with the development of arsenic related diseases including cancers. It has been demonstrated that reactive oxygen species (ROS) and reactive nitrogen species are directly involved in oxidative damage to lipids, proteins and DNA in cells exposed to arsenic, whichcan lead to cell death. On the other hand, many recent studies have provided experimental evidence that arsenic can cause cell damage and death through ROS or RNS signaling pathways. Thus,the generation of ROS and RNS in various biological systems and their roles in induced cellular damage, and activation of oxidative sensitive signaling pathways.
Apoptosis is the process of programmed cell death (PCD) that may occur in multicellular organisms. Programmed cell death involves a series of biochemical events leading to a characteristic cell morphology and death; in more specific terms, a series of biochemical events that lead to a variety of morphological changes, including blebbing, changes to the cell membrane such as loss of membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation (Apoptosis DNA fragmentation). Processes of disposal of cellular debris whose results do not damage the organism differentiate apoptosis from necrosis. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis, in general, confers advantages during an organism's life cycle. For example, the differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. Between 50 billion and 70 billion cells die each day due to apoptosis in the average human adult.
Mitochondrial mediated Apoptosis by mycotoxin:
Several fungal species belonging to the genera Penicillium and Monascus produce secondary metabolites, including the mycotoxin. One of the mycotoxin citrinin has been identified as a contaminant in several types of food, including corn, wheat, rice, barley and nuts, and may cause environmental and human health injury (Blanc P. J,et al., 1995).
Citrinin is reported to be cytotoxic to various mammalian cells, but the mode of cell death in citrinin -exposed cultures has not been fully understood citrinin induced apoptosis through the mitochondria-dependent pathway in human promyelocytic leukemia cells (HL-60) and the process was not directly correlated with cellular oxidative stress.
Apoptotic processes do not occur prior to the blastocyst stage during normal mouse embryonic development (Byrne A. T, et al., 1999) and induction of apoptosis during the early stages of embryogenesis (i.e. by exposure to a teratogen) has been shown to cause embryonic developmental injury (Chan, 2006 and Hsuuw et al., 2005).
The significance of apoptosis:
The development and maintenance of multicellular biological systems depends on a sophisticated interplay between the cells forming the organism, it sometimes even seems to involve an altruistic behaviour of individual cells in favour of the organism as a whole. During development many cells are produced in excess which eventually undergo programmed cell death and thereby contribute to sculpturing many organs and tissues (Meier, 2000). A particularly instructive example for the implication of programmed cell death in animal development is the formation of free and independent digits by massive cell death in the inter digital mesenchymal tissue.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Morphological features of apoptosis:
Apoptotic cells can be recognized by stereotypical morphological changes: the cell shrinks, shows deformation and looses contact to its neighboring cells. Its chromatin condenses and marginates at the nuclear membrane, the plasma membrane is blebbing or budding, and finally the cell is fragmented into compact membrane-enclosed structures, called 'apoptotic bodies' which contain cytosol, the condensed chromatin, and organelles.
During necrosis, the cellular contents are released uncontrolled into the cell's environment which results in damage of surrounding cells and a strong inflammatory response in the corresponding tissue.
Fig-2.Apoptotic versus necrotic morphology
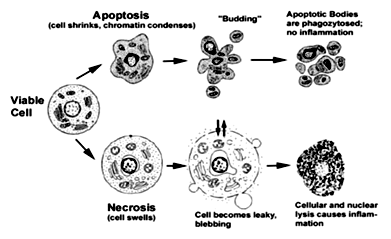
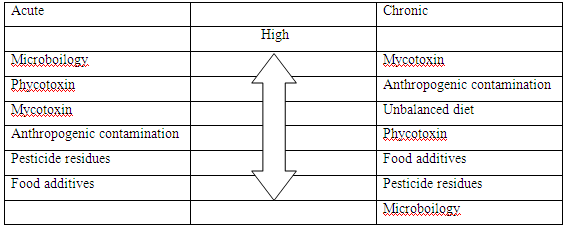
Table-2: Rating health risks from food (Kuiper-Goodman,1998)
Conclusion
• Controls the spreading mycotoxins:
We know these secondary metabolites are so dangerous to both human beings and also other vertebrates. Thus, careful control the mycotoxins should be started and administered by the government of each country through ministries and organizations such as the Ministry of Health, the Ministry of Agriculture, Food and Drug Administration, National Environment Committee Board and Consumer Protection Committee Board. This manage program may be set up by a special administrative committee and the legislative body who regulate the national policy of food safety and the maximum tolerance limits for mycotoxins.
• Aware the farmers towards its bad effects & provides guidelines:
Farmers, middlemen, food and feed factories and exporters will be well educated about mycotoxins and encouraged to prevent and control the contamination of microflora and their health-hazardous mycotoxins in their commodities as much as possible. International organizations such as FAO, WHO and UNEP in the UN system are engaged in providing essential information on various aspects of prevention and control of mycotoxin problems to all the countries. Guidelines for international trade include: a) procedure of sampling and analysis, b) surveillance and food control inspection systems, c) use of contaminated produce in feeding of different animals, d) protocols for detoxification and the quality control of the products. Conferences, symposiums, trainings and workshops on current informations of mycotoxins should be promoted. Low-cost technology for assessment, prevention and control of environmental mycotoxins could be then transferred from developed countries to developing ones (R.L.SemplA.S.Frio, P.A.Hicks, J.V Lozare;RAP Publication-198).
References
• Bayman P, Baker JL "Ochratoxins: a global perspective". Myco Citrininhologia 2006;162 : 215–230.
• Bennett JW, Klich M "Mycotoxins" Clin. Microbiol.2003;16 : 497–516.
• Burdon, R. H., Alliangana, D. and Gill, V. Free Radical Biol. Med. 1995: 18:775-79.
• Bus and Popp, 1987. J.S. Bus and J.A. Popp, “Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally-related compounds” Food Chem. Toxicol. 1987;25:619–62.
• Byrne A. T., Southgate J., Brison D. R., Leese H. J. “Analysis of apoptosis in the preimplantation bovine embryo using TUNEL” J. Reprod. Fertil. 1999;117:97–105.
• Carlberg I, Mannervik B “Glutathione reductase” Methods Enzymol 1985;113: 484–490.
• Carlton, W. W. (1980). Citrinin in "Conference of mycotoxins in animal feeds and grains relatedto animal health" Food and Drug Administration. U.S. Department of Commerce National Technical Informat ion.Cerutti, P. Lancet1994;344:862-863.
• Chan W. H. “Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts”. Hum. Reprod. 2006;21:2985–2995.
• Cornely OA "Aspergillus to Zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infections". Infection 2008; 36:296–313.
• Coultas L, Strasser A.“The role of the Bcl-2 protein family in cancer.” Semin Cancer Biol. 2003;13:115-123.
• Craven, P. A., Pfanstiel, T. and Rubertis, F. R. J. Clin. Invest. 1986 ;77: 850-859.
• Desjardins AE, Proctor RH "Molecular biology of Fusarium mycotoxins". Int. J. Food Microbiol. 2007;119:47–50.
• Harman, D. Mutat. Res.1992;275:257-266.
• Hsuuw Y. D., Chang C. K., Chan W. H., Yu J. S. “Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts.” J. Cell. Physiol.2005;205: 379–386.
• Imlay JA “Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro.” Science,1988;240:640–642.
• Jackson, J. H.,Environ. Health Perspect.1994;102:155-158.
• Khan et al., M.F. Khan, P.J. Boor, B.S. Kaphalia, N.W. Alcock and G.A.S. Ansari, “Hematopoietic toxicity of linoleic acid anilide: importance of aniline.” Fundam. Appl. Toxicol. 1995;25: 224–232.
• Khan et al., M.F. Khan, X. Wu, B.S. Kaphalia, P.J. Boor and G.A.S. Ansari, Acute hematopoietic toxicity of aniline in rats. Toxicol. Lett.1997b;92:31–37.
• Khan et al., M.F. Khan, X. Wu, P.J. Boor and G.A.S. Ansari, Oxidative modification of proteins and lipids in aniline-induced splenic toxicity. Toxicol. Sci.1999a;48:134–140.
• Khan et al., 2003a. M.F. Khan, X. Wu, G.A.S. Ansari and P.J. Boor, Malondialdehyde–protein adducts in the spleens of aniline-treated rats: immunohistochemical detection and localization. J. Toxicol. Environ. Health, Part A 66 (2003a), pp. 93–102.
• Khan et al.,M.F. Khan, X. Wu and J. Wang, Up-regulation of transforming growth factor-β1 in the spleen of aniline-treated rats. Toxicol. Appl. Pharmacol.2003b;187:22–28.
• Lennon SV, Martin SJ, Cotter TG. "Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli". Cell Prolif.1999;24: 203–14.
• Letai A. “Pharmacological manipulation of Bcl-2 family members to control cell death.”
J Clin Invest. 2005;115:2648-2655.
• Mateo R, Medina A, Mateo EM, Mateo F, Jiménez M ."An overview of ochratoxin A in beer and wine". Int. J. Food Microbiol.October 2007; 119:79–83.
• Moss MO. "Fungi, quality and safety issues in fresh fruits and vegetables". J. Appl. Microbiol. 2008;104.
• R.L.Semple, A.S. Frio, P.A..Hicks, J.V Lozare . “Mycotoxins Prevation and Control in foodgrain”. FAO Corporation Document Repository, RAP Publication-1989.
• Sies H and de Groot H. “Role of reactive oxygen species in cell toxicity” Toxicol Lett; 1992;64-65:547-51.
• Totter, J. R. Proc. Natl. Acad. Sci. U.S.A.1980; 77:1763-1767.
• Weitzman, S. A. and Gordon, L. I. Blood,1990;76: 655-663.
• Wiseman H: “Damage to DNA by reactive oxygen and nitrogen species:Role in inflammatory disease and progression to cancer.” Biochem J,1996; 313:17–29.
• Zimmerman, R. and Cerutti, P. Proc. Natl. Acad. Sci. U.S.A.,1984;81: 2085-2087.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE