About Authors:
Aniket Singh*, Poonam Sain, Rekha Singh Saurabh1, Surendra Singh, Kamal Singh Rathore1
*Department of Pharmaceutics, Lachoo Memorial College of Science and Technology (Pharmacy Wing), Shastri Nagar, Jodhpur, 342003 (Raj.), INDIA.
1B.N. Institute of Pharmaceutical Sciences, Udaipur-Raj.313002 INDIA
aniketsingh18@yahoo.com
Abstract
Phytosomes of soy-isoflavones (genistin/daidzin) was formulated to provide good bioavailability for treatment of cancer associated with skin and prostate etc. There are many dosage forms like tablet, capsules, liposomes etc. of isoflavones having anticancer activity available in market but still need for a new dosage form which acts effectively with better bioavailable dose. So the present investigation has been taken up to design, prepare and evaluate phytosomes to meet need of bioavailability and local action. Benefits of this phytosomes showed increase in bioavailability, reduction in gastric irritation by passing first pass metabolism and increase onset of action. Phytosomes were prepared using lecithin as phospholipid and ethanol, acetone etc as solvent system. All formulations were subjected to physic-chemical evaluation. Selected were subjected to stability studies, spectroscopic and biological evaluations.
REFERENCE ID: PHARMATUTOR-ART-1999
Introduction
A soy derived isoflavone has recently attracted much attention of the medical scientific community. This compound was found to be a potent agent in both prophylaxis and treatment of cancer as well as other chronic diseases. The great interest that has focused on genistein led to the identification of numerous intracellular targets of its action in the live cell. At the molecular level, genistein inhibits the activity of ATP utilizing enzymes such as: tyrosine—specific protein kinases, topo-isomerase II and enzymes involved in phsphatidylinositol turnover. Moreover, genistein can act viaan estrogen receptor—mediated mechanism. At the level one step higher, i.e. at the cellular level, genistein induces apoptosis and differentiation in cancer cells, inhibits cell proliferation, modulates cell cycling, exerts antioxidant effects, and inhibits angiogenesis and suppresses osteoclast and lymphocyte functions. These activities make genistein a promising innovative agent in the treatment of cancer. Additionally, genistein health beneficial effects have been shown in osteoporosis, cardiovascular diseases and menopause. Genistein was also successfully used as an immunosuppressive agent both in vitroand in vivo. All these effects at the three biological levels of action need varied genistein concentrations and only some of them are relevant in people consuming soy—rich diet. The others would occur after purified genistein administration at higher doses. The main genistein advantage as a potential drug is its multidirectional action in the live cell and its very low toxicity [1].
Phytosome technology: The flavonoid and terpenoid constituents of plant extracts lend themselves quite well for the direct binding to phosphatidylcholine. Phytosomes results from the reaction of a stoichiometric amount of the phospholipid (phosphatidylcholine) with the standardized extract or polyphenolic constituents (like simple flavonoids) in a non polar solvent [2]. Phosphatidylcholine is a bifunctional compound, the phosphatidyl moiety being lipophilic and the choline moiety being hydrophilic in nature. Specifically the choline head of the phosphatidylcholine molecule binds to these compounds while the lipid soluble phosphatidyl portion comprising the body and tail which then envelopes the choline bound material. Hence, the phytoconstituents produce a lipid compatible molecular complex with phospholipids, also called as phyto-phospholipid complex. Molecules are anchored through chemical bonds to the polar choline head of the phospholipids, as can be demonstrated by specific spectroscopic techniques. Precise chemical analysis indicates the unit phytosome is usually a flavonoids molecule linked with at least one phosphatidylcholine molecule. The result is a little micro sphere or cell is produced. The phytosome technology produces a little cell, whereby the plant extract or its active constituent is protected from destruction by gastric secretions and gut bacteria owing to the gastro-protective property of phosphatidylcholine [3-5].
Differences between liposomes and phytosomes
Likewise phytosomes, a liposome is formed by mixing a water soluble substance with phosphatidylcholine in definite ratio under specific conditions. Here, no chemical bond is formed; the phosphatidylcholine molecules surround the water soluble substance. There may be hundreds or even thousands of phosphatidylcholine molecules surrounding the water-soluble compound. In contrast, with the phytosome process the phosphatidylcholine and the plant components actually form a 1:1 or a 2:1 molecular complex depending on the substance(s) complexes, involving chemical bonds. This difference results in phytosome being much better absorbed than liposomes showing better bioavailability. Phytosomes have also been found superior to liposomes in topical and skin care products [6] (Fig. 1).
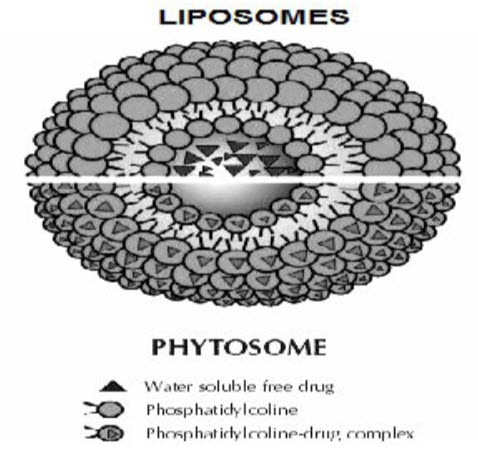
Fig.1. Difference between liposomes and phytosome.
Materials and Methods
Genistin and Daidzin were extracted out by crude soybean sample by extraction method and further dried by spray drying. Phoshpolipid i.e. lecithin and Solvents i.e. ethanol, acetone, acetonitrile and acetic acid wereobtained by S.S. chemicals, Jodhpur. All chemicals were of analytical reagent grade and distilled water was used throughout the study.
Preparation of formulation
Phytosomes are formulated by patented processes in which the standardized extract (having a standardized content of active principles) and/or active ingredients of herbs (like flavor-liganans and terpenoids) are bound to the phospholipids like phosphatidylcholine (PC) through a polar end. The phytosome process produces small cells which protect the valuable components of the herbal extract from destruction by digestive secretions and gut bacteria. They improve transition of constituents from the water phase to the enterocytes of the gut wall and ultimately they reach the circulation. The phyto-active components of these herbal extracts are well suited to direct binding to phosphatidylcholine from soy. PC is also the principle molecular building block of cell membranes and is miscible with both water and oil/lipid mixtures, and is well absorbed orally. Phospholipids are small lipid molecules in which the glycerol is bound to only two fatty acids, instead of three as in triglycerides, with the remaining site is occupied by a phosphate group. Specifically, the choline head of the phosphatidylcholine molecule binds to phytoconstituents while the fat-soluble phosphatidyl portion, comprising the body and tail, then envelopes the choline-bound material. This results in small microspheres or the production of cells known as phytosomes. Thus, phytosomes are also considered as a phyto-lipid delivery system. Phytosomes are prepared by reacting 3–2 moles (preferably with one mole) of a natural or synthetic phospholipid, such as phosphatidylcholine, phosphatidyl-ethanolamine or phosphatidyl-serine, with one mole of phytoconstituents either alone or in the natural mixture in an aprotic solvent, such as dioxane or acetone, in a 1:2 or 1:1 ratio. The optimum ratio of phospholipid to phyto-constituent is 1:1. The complex thus formed can be isolated by precipitation with an aliphatic hydrocarbon or lyophilization or spray drying. Some liposomal drug complexes operate in the presence of water or buffer solution where the phytosomes interact with a solvent with a reduced dielectric constant. The common stages for the preparation of phytosomes are charted in Fig. 2.
Phospholipids
↓
dissolved in organic solvent containing Drug/Extract Hydration
↓
Solution of phospholipids in organic Solvent with drug/extract
↓
Drying
↓
Formation of thin film
↓
Formation of phytosomal suspension
Fig.2. Stages of preparation of phytosomes [13].
Mareno and Lampertico, Jiang et al., Maiti et al. and Maiti et al. have described the methods used for phytosome preparation. Jiang, et al. (2001) have optimized the preparation conditions using a uniform design and step regression and have prepared Herba Epimedii total flavonoids phytosomes (EFP) by means of solvent evaporation and investigated the cumulative dissolution of different ratios of EFP-PVP precipitates by means of dissolution release. The optimized preparation conditions are as follows: solvent-tetrahydrofuran, lecithin to PVP ratio 2.5, temperature 40°C and reaction time-3 hrs. The oil/water apparent partition coefficient of icariin was enhanced more than 4-fold by phospholipid [7-14].
Evaluation of Phytosomes
The behavior of phytosomes in both physical and biological systems is governed by factors such as the physical size, membrane permeability, percentage of entrapped solutes, and chemical composition as well as the quantity and purity of the starting materials. Therefore, phytosomes can be characterized in terms of their physical attributes i.e. shape, size, distribution, percentage drug captured, entrapped volume, percentage drug released and chemical composition.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
a. Different characterization techniques used for phytosomes
- Visualization: -Visualization of phytosomes can be achieved using transmission electron microscopy (TEM) and by scanning electron microscopy (SEM) [15].
- Vesicle size and zeta potential: - The particle size and zeta potential can be determined by dynamic light scattering (DLS) using a computerized inspection system and photon correlation spectroscopy (PCS) [16].
- Entrapment Efficiency: -The entrapment efficiency of a drug by phytosomes can be measured by the ultracentrifugation technique [17].
- Transition Temperature: -The transition temperature of the vesicular lipid systems can be determined by differential scanning calorimetry [18].
- Surface tension activity measurement: -The surface tension activity of the drug in aqueous solution can be measured by the ring method in a Du Nouy ring tensiometer [19].
- Vesicle stability: -The stability of vesicles can be determined by assessing the size and structure of the vesicles over time. The mean size is measured by DLS and structural changes are monitored by TEM [20].
- Drug content: -The amount of drug can be quantified by a modified high performance liquid chromatographic method or by a suitable spectroscopic method [21].
b. Spectroscopic evaluations
To confirm the formation of a complex or to study the reciprocal interaction between the phytoconstituents and the phospholipids, the following spectroscopic methods are used.
- 1H-NMR
The NMR spectra of (+)-catechin and its stoichiometric complex with distearoyl-phosphatidylcholine had been studied by Bombardelli et al. In nonpolar solvents, there is a marked change of the 1H-NMR signal originating from the atoms involved in the formation of the complex, without any summation of the signal peculiar to the individual molecules. The signals from the protons belonging to the flavonoids are to be broadened that the proton cannot be relieved. In phospholipids, there is broadening of all the signals while the singlet corresponding to the N-(CH3)3 of choline undergo an uplift shift. Heating the sample to 60°C results in the appearance of some new broad bands, which correspond mainly to the resonance of the flavonoid moiety.
- 13C-NMR
In the 13C-NMR spectrum of (+)-catechin and its stoichiometric complex with distearoyl-phosphatidylcholine, particularly when recorded in C6D6 at room temperature, all the flavonoid carbons are clearly invisible. The signals corresponding to the glycerol and choline portion of the lipid (between 60–80 ppm) are broadened and some are shifted, while most of the resonances of the fatty acid chains retain their original sharp line shape. After heating to 60°C, all the signals belonging to the flavonoid moieties reappear, although they are still very broad and partially overlapping.
- FTIR
The formation of the complex can be also be confirmed by IR spectroscopy by comparing the spectrum of the complex with the spectrum of the individual components and their mechanical mixtures. FTIR spectroscopy is also a useful tool for the control of the stability of phytosomes when micro-dispersed in water or when incorporated in very simple cosmetic gels. From a practical point of view, the stability can be confirmed by comparing the spectrum of the complex in solid form (phytosomes) with the spectrum of its micro-dispersion in water after lyophilization, at different times. In the case of simple formulations, it is necessary to subtract the spectrum of the excipients (blank) from the spectrum of the cosmetic form at different times, comparing the remaining spectrum of the complex itself [22-24].
c. In-vitroand In-vivo evaluations: -
Models of in-vitro and in-vivo evaluations are selected on the basis of the expected therapeutic activity of the biologically active phytoconstituents present in the phytosomes. For example, in-vitro anti-hepatotoxic activity can be assessed by the antioxidant and free radical scavenging activity of the phytosomes. For assessing anti-hepatotoxic activity in-vivo, the effect of prepared phytosomes on animals against thioacetamide-paracetamolor alcohol- induced hepatoxicity can be examined. Skin sensitization and tolerability studies of glycyrrhetinic acid-Phytosome® ointment, a commercial product, describe the in vivo safety evaluation methodology. Filburn et al. studied the bioavailability of a silybin-phosphatidylcholine complex in dog models to examine the pharmacokinetic parameters of this new complexed form [25-27].
Conclusion
The poor absorption and the poor bioavailability associated with the polar phytoconstituents limits its use. Phospholipid based drug delivery system have been found promising for better and effective delivery of drug and can enhance the rate and extent of drug absorption across the lipoidal bio-membrane. Phytosomes are novel formulations which offer improved bioavailability of hydrophilic flavonoids and other similar compounds through the skin or gastrointestinal tract. They have many distinctive advantages over other conventional formulations. The formulation methodology for phytosome is simple and can be easily upgraded to a commercial scale. The characterization methodologies and analytical techniques are well established for this type of novel formulation. Many patents are already approved for innovative formulations, processes and applications of phytosomes. Presently phytosomes are used primarily in cosmetics to deliver water soluble substances to the skin. The technology can effectively deliver the product by topical and oral route. Phytosomes enables pharmaceutical manufacturers to provide new pharmaceutical products using water soluble drugs and provides new developments in medical industry.
Acknowledgements
I am very thankful to principal and all teachers of Lachoo Memorial College of Science and Technology (Pharmacy wing) for providing necessary laboratory facilities to carry out this work with great ease and precision. I am profusely thankful to Dr. K.S. Rathore , Asso.Prof. BN Institute of Pharmaceutical Sciences, Udaipur for his continuous, perennial and incessant help throughout the course of the study
References
1.Krzysztof Polkowski and Alexander P Mazurek, “Biological properties of Genistein: A review of in-vitro and in-vivo data”, Drug institute, 30/34, Chelmska, 00-725, Warsaw, Poland, 130-155.
2.E Bombardelli, SB, Curri, R, Loggia Della, et al. Complexes between phospholipids and vegetal derivatives of biological interest. Fitoterapia. 1989, 60: 1-9.
3.Bombardelli E. Phytosome: New Cosmetic Delivery System. BollChim Farm. 1991; 130:431-38.
4.Bombardelli E, Spelta M. Phospholipid-Polyphenol Complexes: A New Concept in Skin Care Ingredients. Cosm & Toil.1991; 106:69-76.
5.Murray D. Phytosomes- Increase the Absorption of Herbal Extract [online]. 2008 [cited 2008 Sep 28]. doctormurray.com/articles/silybin.htm.
6.Phytosomes: A Technical Revolution in Phytomedicine [online]. 2010 [cited 2010 Mar 22]. Available from: URL: indena.com.
7.C Marena, M Lampertico. Preliminary clinical development of silipide: A new complex of silybin in toxic liver disorders. Planta Med., 1991, 57: A124-A125.
8.U Citernesi, M Sciacchitano. Phospholipids/active ingredient complexes. Cosm. & Toil. 1995, 110: 57-68.
9.MT Murray. Phytosomes: Herbal Support – Increase the Absorption of Herbal Extracts, Available at doctomurray.com/articles/silybin.htm. 2004.
10.NK Jain. Liposomes as drug carriers, controlled and novel drug delivery, 1st edition, CBS publisher, 2005, 308, 321-326.
11.YN Jiang, Z. P. Yu, Z. M. Yan, et. al. Preparation of herba epimedii flavanoid and their pharmaceutics. Zhongguo Zhong Yao, 2001, 26: 105-108.
12.K Maiti, K Mukherjee, A Gantait, et al. Curcumin phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. pharm., Sept. 2006. (In Press).
13.K Maiti, K Mukherjee, A Gantait, et al. Enhanced therapeutic potential of naringenin-phospholipid complex in rats. J. Pharm. Pharmacol., 2006, 58: 1227-1233.
14.X Yanyu, S Yunmei, C Zhipeng, et al. The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats. Int. J. Pharm., 2006, 307: 77-82.
15.GMM Maghraby El, AC Williams, BW Barry. Oestradiol skin delivery from ultra-deformable liposomes: refinement of surfactant concentration. Int. J. Pharm., 2000, 196: 63-74.
16.DW Fry, JC White, ID Goldman. Rapid secretion of low molecular weight solutes from liposomes without dilution. Anal. Biochem. 1978, 90: 809-815.
17.Liposomes: A Practical Approach, Preparation of liposomes and size determination, New RRC (Ed.), Oxford University Press, 1990, 36-39.
18.G Cevc, A Schatzlein, G Blume. Transdermal drug carriers: basic properties, optimization and transfer efficiency in case of epicutaneously applied peptides. J. Control. Release, 1995, 36: 3-16.
19.BAI V Berge, VAB Swartzendruber, J. Geest. Development of an optimal protocol for the ultra-structural examination of skin by transmission electron microscopy. J. Microsc., 1997, 187: 125-133.
20.N Dayan, E Touitou. Carrier for skin delivery of trihexyphenidyl HCl: ethosomes vs liposomes. Biomaterials, 2002, 21:1879-1885.
21.RM Facino, M Carini, G Aldini, et al. Free radicals sea action and anti-enzyme activities of procyanidines vitis vinifera-a mechanism for their capillary protection. Arzneim. Forsch, 1994, 44: 592-601.
22.A Semalty, M Semalty, R Singh, MSM Rawat. Phytosomes in herbal drug delivery. Indian drugs, 2006, 43: 937-946.
23.E Bombardelli, G Mustich. Bilobalide-phospholipid comlex, their uses and formulation containing them. U. S. Patent No. EPO-275005, 1991.
24.S Abrol, A Trehan, OP Katare. Comparative study of different silymarin formulations: formulation, characterization and in vitro/in vivo evaluation. Current Drug Delivery, 2005, 2: 45-51.
25.A Comoglio, A Tomasi, S Malandrino, et al. Scavenging effect of silipide-A new silybinphospholipid complex, on ethanol-derived free radicals. Biochem. Pharmacol. 1995, 50: 1313-1316.
26.U Delgi, SD Urbino. Tolerability and cutaneous sensitization study in healthy volunteers after topical application of the product glycyrrhetinic acid-Phytosome® ointment. Unpublished data submitted by CTFA, 2004, 36: 2.
27.CR Filburn, R Kettenacker, DW Griffin. Bioavailability of a silybin-phosphatidylcholine complex in dogs. J. Vet. Pharmacol. Ther. 2007, 30: 132-138.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









