{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Kavitha.A*, Narendra Babu.A, Sathish Kumar M, Veena Kiran.S
Department of pharmacology
Chalapathi institute of pharmaceutical sciences,
Lam, Guntur-522034, Andhra Pradesh
andugulakavitha@gmail.com
ABSTRACT
Background:
Hyperandrogenism and insulin resistance are the main manifestations of polycystic ovary syndrome (PCOS), which appears to be caused by exposure to androgenized models have developed and investigated to study the etiology of polycystic ovary syndrome.
Objective:
To evaluate the modulatory effects of Commiphora wightii (C.wightii) resins in response to hyperandrogenism in polycystic ovary syndrome.
Method:
The animals were divided 18 adult (5-6 months old) wistar rats in to 4 groups the PCOS model was induced by daily administration of dehydroepiandrosterone (DHEA) 6mg/Kg in sesame oil p.o., up to 15 days and the rescue groups were take daily with metformin and C.wightii resin ethanolic extract 100mg/ kg in addition to DHEA. Serum glucose levels measured and steroid hormone levels were measured by fully automated bidirectionally interfaced chemi luminescent immunoassay. Samples were stained with hematoxylin and eosin for histological morphology.
Results:
The obtained results related to DHEA induced PCOS a significant (P<0.05) increase in hormone profile (estradiol, testosterone, progesterone, luteinizing hormone, follicle stimulated hormone) in PCOS rats in adult rats than the rescue groups. Furthermore glucose levels significantly (<0.05) elevated in PCOS rats compared with the other groups. The test treated ovaries had lower number of follicles compared to DHEA control group and similar to that of the control group than the standard.
Conclusion:
Commiphora wightii resin has a potential role in reducing DHEA induced PCOS by reducing the morphological abnormalities of the ovarian follicles and normal hormone levels in adult rats.
INTRODUCTION:
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders among women affecting 5– 10%of those of reproductive age. PCOS is characterized by hyperandrogenism, insulin insensitivity, and chronic anovulation.
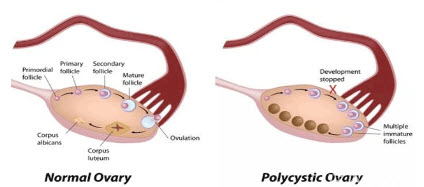
Figure no 1: difference in normal ovary and PCOS ovary
Research over the last few decades has established that Polycystic ovary syndrome is an important metabolic disorder, It has been proposed women who have mild hyperandrogenism and an isolated ultrasonic finding of polycystic ovaries but whose ovulatory function is maintained exhibit a mild form of Polycystic ovary syndrome. These women may be susceptible to developing the syndrome as well. Increased luteinizing hormone (LH) and increased insulin levels mainly amplify the intrinsic abnormality of their steroidogenesis. In PCOS, excess androgen activity may alter gonodotropins-induced estrogen and progesterone synthesis in the follicles. Normally, testosterone and androstenedione are converted to estradiol and estrone, respectively, with help of P450 aromatase, which plays an important role in the ovary’s hormonal balance. However, decreased activity of this enzyme results in the increased ovarian androgen production and development of the polycystic ovary condition. The etiology of Polycystic ovary syndromeis multi factorial and is attributed to genetic factors as well as primary defects of the hypothalamic-pituitary unit, micro-environment of the ovary such defects of intra ovarian molecules involved in paracrine /autocrine regulation, such as insulin-like growth factor-I, and an overactive adrenal gland. Ovarian functions are regulated by hormonal and neural signals. Hormonal signals regulating ovarian functions arise from the pituitary, adrenal, ovaries, thymus and thyroid; while neural signals arrive to the ovaries through the superior ovarian nerve (SON), the plexus ovarian nerve (OPN) and the vagus nerve. Neural signals modulate the effects of hormonal signals on the follicular, luteal and interstitial compartments. Several androgenized animal models have been developed and investigated to determine the etiology of polycystic ovary syndrome. Slight differences among the models reflect differences in the timing and duration of the androgen administration, as well as the amount of hormone administered. Nearly all animals administered androgen during fetal life exhibit polycystic ovary(PCO) and show endocrine abnormalities similar to polycystic ovary syndrome (PCOS). In a report on the effects of androgen administration to immature rats, short-term treatment caused PCO morphology. The similarities in key steps of mammalian reproduction make animal models a attractive for studying the pathogenesis of this syndrome. Polycystic ovary (PCO) can be induced in immature rats by daily injection of dehydroepiandrosterone (DHEA). The polycystic ovaries of the DHEA-treated animals are steroidogenically more active than those of controls, raising the possibility that the DHEA acts directly on the ovary in addition to an action on the pituitary-hypothalamus axis. In an effort to produce a model to study PCOs, DHEA was administering to immature rats, which resulted in ovarian cystic and hormonal changes. This model exhibits many of the salient features of human PCOS. Under these conditions, prepubertal female rats develop cysts and become anovulatory and acyclic. Follicles after DHEA treatment underwent atresia or exhibited various stages of cystogenesis. Complete atresia or cystogenesis may complete the pathological transformation into a follicular cyst. Metformin is an oral insulin sensitizer widely used for type 2 diabetes, and has been recommended for the treatment of anovular PCOS, mainly by reducing serum insulin levels and insulin resistance and improving ovulatory function. Given the fact that metformin has great potential in attenuating the progress of fibrosis in various organs , we hypothesized that DHEA-induced fibrosis could be decreased by metformin treatment, which may account for its clinical efficacy for PCOS patients, as it improves the endocrine/ clinical aspects of PCOS and contributes to improved glucose profiles . This may also be a mechanism for its effectiveness in the clinical trials. In summary, we hypothesized that the level of fibrosis was significantly higher in ovarian and uterine tissues in DHEA-induced PCOS rats. Commiphora wightii belongs to Barseracea family of plant kingdom. It has a resinous secretion known as gug-gul. Guggul is one of the most valuable remedies in one of the traditional system of medicine like the ayurvedic medicine. Its usage backs to over 2500 years. Guggul has been used as antidiabetic, antihyperlipidemic, anti-osteoarthritic, anti-inflammatory, antispasmodic, sedatives, antiseptic, astringent, carminative, emmenagogue, expectorant, diaphoretic, thyroid stimulant, antiobesity, diuretic, anthelminitic, and demulcent in traditional medicine. It has no well-defined chemical composition due to its very complex nature. Phytochemical investiga-tion of guggul indicates the presence of sugars (fructose, sucrose), amino acids, oil and several steroids. An Ethanolic extract of guggul two important bioactive compounds E&Z-guggulsterones known as guggulpid,
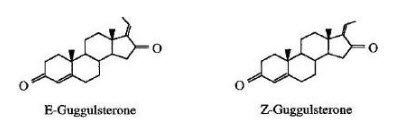
Structure of E&Z-Gugguldterons (cis-and-trans-4,17(20)-pregnadiene-3, 16-dione)
The aim of the present study was to investigate the effects of DHEA administered during prepubertal period on ovarian follicular development and hormonal profile. Consequently, the protective effects of Commiphora wightii against the deleterious effects of DHEA toxicity on ovarian follicular development, hormonal profile were investigated and could be attenuated by treatment with metformin.
MATERIALS AND METHODS:
Collection of plant material:
Commiphora wighti,was collected from the fields of Guntur. During the month Nov-Dec 2013 the material was botanically identified and confirmed by Botanist. After resins of Commiphora wightii were collected.
Preparation of crude extracts:
Resins of guggul were extensively washed under tap water followed by washing with sterilized distilled water. They were further air-dried on filter paper at room temperature and then powdered with the help of sterilized pestle and mortar under aseptic condition. Further air-dried powders (30g) of the resins wereextracted with ethanol for three days by using Sox let apparatus at 30ºC. After that the extracts were concentrated with rotary evaporator and dried in vacuum. The extract washed with 3N HCl and fractions were taken for study.
Table 1: Phytochemical analysis of resins of Commiphora wightiiethanolic extract
|
S.No: |
Test |
Ethanolic extract Observation |
|
1. |
Mayer’s test |
+ |
|
2. |
Dragandroff’s test |
+ |
|
3. |
Protein |
+ |
|
4. |
Tannin |
+ |
|
5. |
Carbohydrate |
+ |
|
6. |
Reducing sugar |
+ |
|
7. |
Flavonoids |
+ |
|
8. |
Saponins |
+ |
|
9. |
Phenol |
+ |
|
10 |
LB test |
+ |
Polycystic ovary syndrome against activity of Commiphora wightii:
The study was carried out using adult (5-6 months old) wistar rats, with body weights of 20–45 g. Immature rats were divided in to four groups contains six animals in each group. Group I serve as control and received regular rat food and drinking water ad libitum. Control animals were receive orally with 0.2 ml of sesame oil, Group II serves as DHEA control and received regular rat food and drinking water ad libitum. And DHEA control was receiving orally DHEA (6 mg/100 g body weight/0.2 ml sesame oil). Group III serves as standard and animal receive Metformin 2mg metformin /100g body weight/day in sesame oil and after 30 minutes DHEA (6 mg/100 g body weight/0.2 ml sesame oil) and Group IV serve as test and this group animals receives C.wightii ethanolic extract 100 mg in sesame oil and after 30 minutes DHEA (6 mg/100 g body weight/0.2 ml sesame oil) from 1st day to 15th day. All drug and extract were given once daily by oral route.Body weight of all animals were monitored initially and the 16th day.Groups of rats were killed on day16; Ovaries from each group of rats were processed for light and electron microscopy and for follicular or cystic fluid hormone analysis.
RESULTS
PHYSICAL PARAMETERS
Table 2: Effect of C.wightii on body weight of adult rats in DHEA induced polycystic ovary syndrome
|
S.No |
Group |
Mean ± SEM (n=6) |
||
|
Initial body weight in gms |
Final body weight in gms |
Difference in body weight in gms |
||
|
I |
Control |
234.36±0.447 |
256.3±0.4728 |
22.15 ± 0.8440 |
|
II |
DHEA Control |
233.78±0.421 |
284.2±1.9549 |
51.15 ±0.5991 |
|
III |
Metformin |
233.51±0.232 |
273.35±0.3253 |
39.65 ±0.0314 |
|
IV |
Test |
234.11±0.291 |
263.2±0.3803 |
29.58 ±1.8992 |
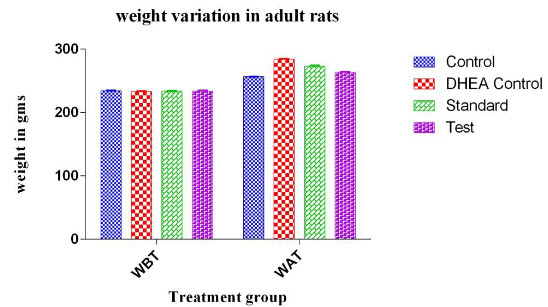
WAT: weight before treatment, WAT: weight after treatment
Figure no 2
Table 3: Effect of C.wightii on ovaries weight of adult rats in DHEA induced polycystic ovary syndrome
|
S.No |
Treatment group |
Ovary weight in mg Mean ± SEM (n=6) |
|
|
Left ovary |
Right ovary |
||
|
1 |
Control |
76.78±3062 |
77.76±04255 |
|
2 |
DHEA Control |
91.85±0.4041 |
91.85±0.3340 |
|
3 |
Standard |
85,15±0.4090 |
84.95±0.6333 |
|
4 |
Test |
76.85±0.3675 |
76.23±0.3955 |
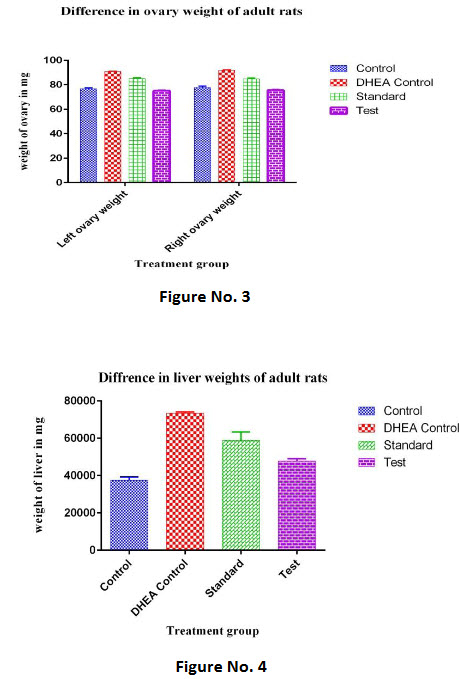
Table 4: Effect of C.wightii on liver levels of adult rats in DHEA induced polycystic ovary syndrome
|
S.No |
Treatment group |
Mean ± SEM(n=6) for liver Weight (mg) |
|
1 |
Control |
3144.2± 202.12 |
|
2 |
DHEA Control |
6209.8± 140.85 |
|
3 |
Standard |
4890.9± 56.24 |
|
4 |
Test |
4196.6±15.48 |
BIOCHEMICAL PARAMETERS
Table 5: Effect of C.wightii on glucose levels of adult rats in DHEA induced polycystic ovary syndrome
|
S.No |
Treatment group |
Serum glucose levels mg/ dL |
|
1 |
Control |
76.24 ± 0.413 |
|
2 |
DHEA Control |
291.32 ± 2.044 |
|
3 |
Standard |
76.40 ± 0.468 |
|
4 |
Test |
140.65 ± 2.257 |
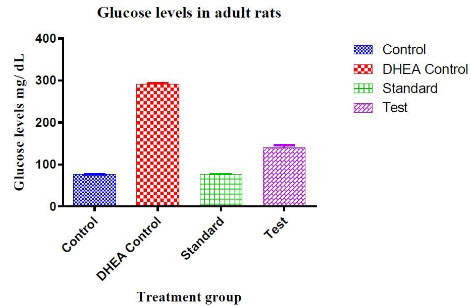
Figure no 5
Table 6: Effect of C.wightii on hormone levels of adult rats in DHEA induced polycystic ovary syndrome
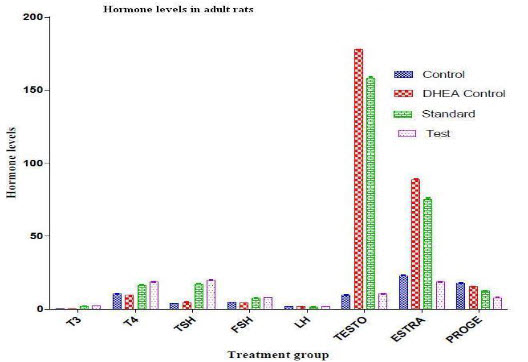
Figure no 6:
triidotyronine (T3), thyroxin (T4), thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone (TESTO), estradiol (ESTRA), progesterone (PROGE) hormone levels
HISTOPATHOLGY OF OVARIES
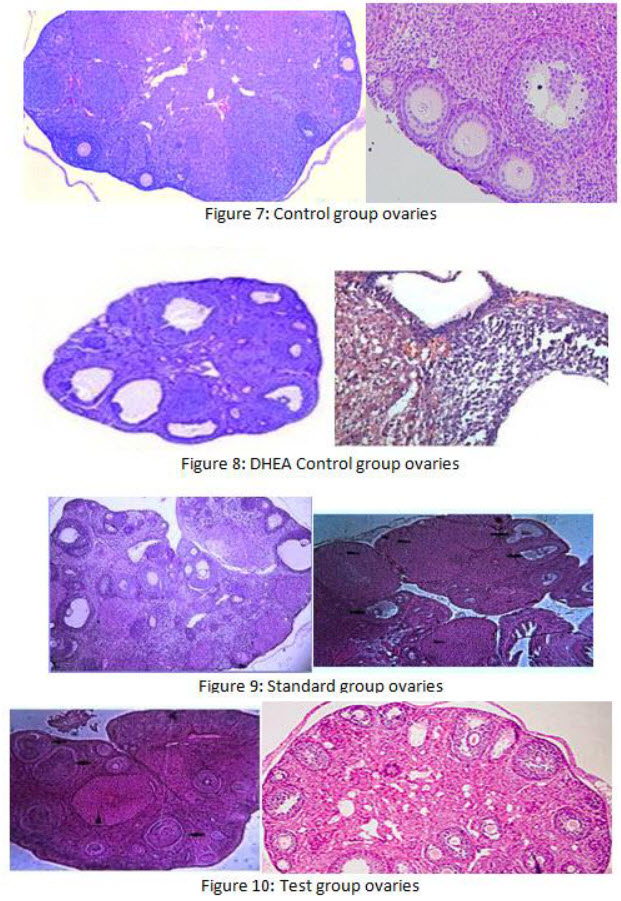
The general architectures of the ovaries in the control and test (C.wightii 100 mg/kg) given rats were similar with normal rat ovaries. Exhibited changes in ovarian morphology, such as increased size, thickened capsule, cystic graffian follicle. An inner layer of granulosa cells with uniform round nuclei and little cytoplasm lines follicle cysts and an inner layer of theca interna cells, which are small and spindle-shaped. The luteinized layers were observed in PCOS model rats. There were no cystic graffian follicle and histopathological changes in all PCOS+C.wightii 100 mg/kg group.
STATISTICAL ANALYSIS:
All values of results are presented as mean ± standard error of mean (SEM). The statistical analysis involving two groups was evaluated by means of Student’s t-test, whereas one way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison post test was used for statistical comparison between control, standard and test treated groups. The results were expressed as mean ± S.E.M (standard error of Means). P value less than 0.05. Statistical significance was accepted at the values.
DISCSSION:
Polycystic Ovary Syndrome is one of the most common endocrinopathy affecting women8 it called as the Stein–Leventhal syndrome9. In 1935 Irving F. Stein and Michael L. Leventhal described a symptom complex due to anovulation10. Oligomenorrhea, hirsutism and obesity together with enlarged polycystic ovary (PCO) were the diagnostic criteria of polycystic ovary syndrome is one. It is now accepted that this problem is arising from persistent anovulation with a spectrum of etiologies and clinical manifestations11.
Hyperandrogenism and insulin resistance are the main manifestations of polycystic ovary syndrome (PCOS), which appears to be caused by exposure to and rogenized models have developed and investigated to study the etiology of polycystic ovary syndrome.
The exact prevalence of Polycystic Ovary Syndrome is not known as the syndrome is not defined precisely. Globally, prevalence estimates of PCOS are highly variable, ranging from 2.2% to as high as 26%.The estimated prevalence in women of reproductive age is 5-10%. Under the new criteria (Rotterdam-2003), the prevalence among the general female population will raise up to 10%12.
Herbal therapy has reached a turning point. It is fighting to be recognised as a science-a particular field with its own identity. It has become necessary to show that herbal therapy can match other fields of medicine in the thoroughness of its scientific work and its practical use. Benefit of herbal therapy compared to conventional therapy is that herbal therapy is safe with lesser side effects and presence of multiple active compounds in medicinal herbs altogether provides a potentiating effect4-5.
Dehydroepiandrosterone induced polycystic ovary syndrome model was used to assess the polycystic ovary syndrome against activity in wistar rats. Both immature rats were divided in to four groups congaing six animals in each group. Group I serve as control and received regular rat food and drinking water ad libitum. Control animals were receive orally with 0.2 ml of sesame oil, Group II serves as DHEA control and received regular rat food and drinking water ad libitum. And DHEA control was receiving orally DHEA (6 mg/100 g body weight/0.2 ml sesame oil). Group III serves as standard and animal receive Metformin 2mg metformin /100g body weight/day in sesame oil and after 30 minutes DHEA (6 mg/100 g body weight/0.2 ml sesame oil) and Group IV serve as test and this group animals receives C.wightii ethanolic extract 100 mg in sesame oil and after 30 minutes DHEA (6 mg/100 g body weight/0.2 ml sesame oil) from 1st day to 15th day. All drug and extract were given once daily by oral route.
Assessment of polycystic ovary syndrome against activity:
Measurement of body weight:
For adult rats the change of body weight of each animal was measured by subtracting the initial body weight of animal on the day of 0 from the weight of animal on 16th day. There was a significant decrease in the body weights of the test drug treated animals (P< 0.0106) the body weight reduction in adult rats was found to significant.
Measurement of ovary weight:
For adult rats the ovary weight of each animal was measured on 16th day. There was a significant decrease in the ovary weights of the test drug treated animals. (P< 0.0001)The weight reduction of ovaries in adult rats was found to significant.
Measurement of liver weight:
For adult and rats the liver weights of each animal was measured on 16th day. There was a significant decrease in the liver weights of the test drug treated animals. (P< 0.0001) The weight reduction of liver in adultrats was found to significant.
Measurement of glucose levels:
For adult rats the glucose levels of each animal was measured on 16th day. There was a significant decrease in the glucose levels of the test drug treated animals. (P< 0.0001) the reduction of glucose levels in adultrats was found to significant.
Serum analysis:
After the experimental period, blood was collected form heart puncture and analyzed. In case of immature rats the triidotyronine (T3) , thyroxin (T4), thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH) levels were increased, where as luteinizing hormone (LH), testosterone (TESTO), estradiol (ESTRA), progesterone (PROGE) hormone levels were decreased. There was a significant decrease in the glucose levels of the test drug treated animals. (P< 0.0031) the reduction of glucose levels in both adult and immature rats was found to significant.
Histological Procedure for Light Microscopy:
The sections were stained in H&E (heamotoxylin and eosin) staining and photographs were taken using a light microscope with a camera attachment (Nikon Eclipse E600, Japan) the no of follicles cells were increased in the immature test (100 mg/Kg C.wightii ethanolic extract) treated groups. There was a significant increase in the ovarian follicle in the adult rats.
Metformin, a potential drug for PCOS treatment, has recently been proposed to have an anti-fibrotic effect. C.wightii can be explained by a mechanism antihyperglycemic, insulinogenic and enhanced insulin sensitizing activities.Several chemical components such as diterpenes, sterols, steroids, esters and higher alcohols have been identified in .C.wightii Oleoresin of .C.wightii is a mixture of several steroid lipids. Of these steroids, Z- guggulsterone and E-guggulsterone are the effective. Guggulsterone in the body are easily reduced to guggulsterols which behave as the antioxidant properties of guggulsterols could be explained by the fact that their hydroxyl groups are present at α-positions of double bonds, similar to antioxidant vitamins, and are soluble in lipids. The steroid structure also contains H, CH3 and O bond. The present study indicated that the extract of .C.wightiinotably decreased androgen levels in blood DHEA- induced PCOS in immature rats and adult rats. In agreement with the present results, the PCOS against effect of C.wightii has been experimentally proved in induced PCOS in rats.
CONCLUSION
The findings suggest that ethanolic extract of C.wightii serve as a potent agent on PCOS and seem to be promising for the development of phytomedicines for PCOS effect in PCOS.
ACKNOWLEDGEMENT
One of the author is thankful to Professor Rama Rao. Nadendla, principal, Chalapathi Institute of Pharmaceutical Sciences for providing required facilities to carry out the research work.
REFERENCES:
1. Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R; Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord; 2002; 26(7); 883-896.
2. Glueck CJ, Phillips H, Cameron D, Sieve-Smith L, Wang P; Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reduce first trimester spontaneous abortion: a pilot study; Fertil Steril; 2001; 75(1); 46-52.
3. Laura Borgelt; What Pharmacists Need to Know About Polycystic Ovary Syndrome (PCOS). 2011
4. Pratik Kumar Chatterjee , P.Prasanna Mithra , Rahul Pal , Poulomi Chatterjee , B.Unnikrishnan , N.A.Vinodini , Aparna Tripathi , V.B.Suman , Anish Singha, Sheila R. Pai; Epidemiological correlates among women with Polycystic ovary syndrome in South Indi;a int. J. Curr. Res. Aca. Rev; 2014; 2(9); 181-186.
5. Line Sveberg Røste, Erik Taubøll, Jouko I.T. Isojärvi , Aasmund Berner, Kjell Andersen Berg, Arto J. Pakarinen , Ilpo T. Huhtaniemi , Mikael Knip, Leif Gjerstad; Gonadal morphology and sex hormones in male and female Wistar rats after long-term lamotrigine treatment; Seizure 2003; 12(8); 621–627
6. Michael T. Sheehan, MD; Polycystic Ovarian Syndrome: Diagnosis and Management Clinical Medicine & Research Clin Med Res. 2004 Feb; 2(1): 13–27.
7. Bernier, Danielle, "Polycystic Ovary Syndrome: Pathogenesis, health consequences, and treatment of PCOS in relation to insulin resistance" (2012). Honors Theses. Paper 3.
8. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R; Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078-3082.
9. merckmanuals.com/professional/gynecology-and-obstetrics/menstrual-abnormalities/premature-ovarianinsufficiency- or-failure
10. unboundmedicine.com/merckmanual/view/Merck-Manual/553926/all/Polycystic_Ovary_Syndrome_ PCOS
11. merckmanuals.com/home/women-s-health-issues/menstrual-disorders-and-abnormal-vaginal-bleeding
/polycystic-ovary-syndrome
12. Text book of applied therapeutics by Koda-Kimble 10th eddition
13. “The wealth of India”. First supplement series, raw materials, CSIR, 1956 (VI) L-M: 323-325.
14. C.S.Shah and J. S. Qadry. “Text book of Pharmacognosy”. B.S.Shah Prakasan, 1983; Ahmedabad. 1-15.
15. Buenz.et al. “Techniques: Bio prospecting historical herbal texts by hunting for new leads in old tomes”. Trends in Pharmacological Sciences: 2004; 25:494-498.
16. Anonymous. Zanzibar Traditional and Alternative Medicine Policy, 2008.
17. Weiss RF. Weiss’s herbal medicine. Thieme; 2001.
18. Benzie IF, Galor SW. Herbal Medicine: Biomolecular and Clinical Aspects. CRC Press; 2011. p. 7.
19. Muzler, J. Bohlmann. “The Role of Natural Products in Drug Discovery: Tools for drug discovery”. Ernst Schering Research Foundation, Springer – Verlag berlin, Heidelberg 2000 – New York: 5-6.
20. Acupuncture in Polycystic Ovary Syndrome: Current Experimental and Clinical Evidence E. Stener-Victorin, E. Jedel_ and L. Manneras Journal of Neuroendocrinology 20, 290–298.
21. Natural Remedies for Polycystic Ovarian Syndrome (PCOS): A Review Priyanka Kantivan Goswami, Dr. Anubha Khale, Sunita Ogale Int.J.Pharm.Phytopharmacol.Res. 2012, 1(6): 396-402.
REFERENCE ID: PHARMATUTOR-ART-2383
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 1 Received On: 19/08/2015; Accepted On: 02/09/2015; Published On: 01/01/2016 How to cite this article: Kavitha A, Narendra BA, Sathish KM, Veena KS; Evaluation of effects of Commiphora Wightii in Dehydroepiandrosterone (DHEA) induced Polystic Ovary Syndrome (PCOS) in Rats; PharmaTutor; 2016; 4(1); 47-55 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









