{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Khushbu Patel1, Divya chauhan1, Rabiya Sodha1, Kinjal Vadhwani1, Kapil Daxini2
Department of pharmacy
1Parul Institute of Pharmacy, Waghodiya, Limda, Vadodara, Gujarat
2Sigma Institute of Pharmacy, Bakrol, Vadodara, Gujarat
ABSTRACT
Proteins and peptides are mostly desirable in many diseased states. Most widely employed method of administration for therapeutic proteins and peptides is Parenteral route. Requirement of repeated injections is because of short in vivo half-life response in poor patient compliance. Non-invasive drug delivery routes such as nasal, transdermal, oral, and pulmonary offer several benefits over parenteral administration. Low permeability and intrinsic physicochemical properties across biological membrane limits protein delivery via adjective routes. Delivering through nanostructured delivery carriers is one of the method to improve protein and peptide absorption. Polymeric nanoparticles (NPs) have illustrated significant benefits over other delivery systems. This article summarizes the application of polymeric NPs for protein and peptide drug delivery following nasal, oral, ocular, pulmonary, transdermal, and parenteral administrations. The aim of review is to highlight and explore technological developments in the field of soft matter nanocarriers for the delivery of proteins and peptides via the eye, the nose, the skin, and the lungs and to provide insights in advantages, practicability and limitations of recent advances.
Reference Id: PHARMATUTOR-ART-2673
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 7, Issue 07 Received On: 22/05/2019; Accepted On: 27/06/2019; Published On: 01/07/2019 How to cite this article: chauhan, D., Patel, K., Sodha, R., Vadhwani, K. and Daxini, K. 2019. Dermal Delivery of Protein and Peptides: Recent advances and clinical outcome. PharmaTutor. 7, 7 (Jul. 2019), 21-31 |
INTRODUCTION
The continuous growth in biotechnologically produced pharmaceuticals, reflect increased demand for bio-macromolecules as new therapeutic compounds. The global sales of biologics reached US $163 billion in 2012 and accounted for over 70% of the worldwide revenue. Especially, In the treatment of severe and highly heterogenic diseases like autoimmune diseases. Bio-macromolecules are having high specificity and potency than small molecule drugs, so that they are more effective. One ideal success story is trastuzumab (Herceptin®) which is widely used for the treatment of HER2-positive breast cancer. This drug blocked the human epidermal growth factor receptor 2 by selective binding which is over expressed in about 25% of breast cancer patients. Another successful outcome for the treatment of chronic inflammatory diseases is the tumor necrosis factor α inhibitor adalimumab. In past, its sales increased to $ 4.6 billion due to increased application in dermatology. Other successful examples are the vascular endothelial growth factor(s) with bevacizumab or aflibercept (Zaltrap®) or antibodies for the treatment of colorectal cancer targeting the epidermal growth factor receptor with cetuximab (Erbitux®). The concept of selective biomacromolecules was improved and adapted for many diseases allows dreaming about actual personalized medicine.
From the beginning, due to the specific physicochemical properties of biomacromolecules are challenge for researcher and pharmaceutical companies. Biomacromolecules have complex structure with high molecular weight (between 300 and 1,000,000 Da) and amphiphilic properties. Moreover, biopharmaceuticals are susceptible to stability problems like aggregation and denaturation that can occur during administration, story and manufacturing. If a protein is subjected to agitation, changes in pH, temperature, its secondary structure can easily be interrupted which activates the unfolding of the amino-acid chain and induces aggregation and denaturation and ultimately it reduce the biological activity.
Like Intramuscular, intravenous, or subcutaneous administration having a short in vivo half-life and rapid clearance from the human body. Due to exposure to proteases and peptidases which are expressed abundantly in the systemic circulation and human body organs, the administered proteins can undergo degradation (Fig. 1). Repeated injections of high doses are inevitable to maintain a therapeutic level which triggers adverse effects such as immunogenicity. Based on these, the topical application of proteins or peptides appears an alternative route of administration to reduce systemic side effects, and increase the therapeutic efficacy.
Finally, after implementation of the biomacromolecule has to control several biological membranes to reach the target site (Fig. 1).
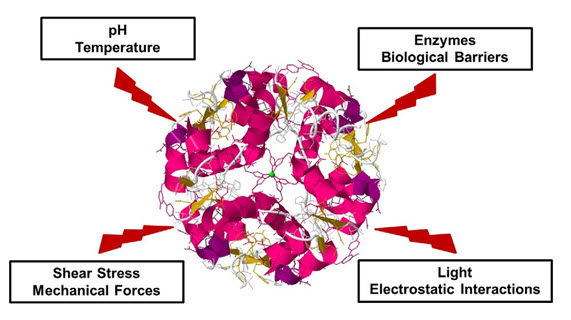
Figure 1. Degradation of protein by environmental factors
Many research efforts are conducted to overcome their limitations and to enable sufficient and effective biomacromolecule delivery. In this regard, smart drug delivery systems need to ensure both the maintenance of the biomacromolecule structure, activity, efficient and targeted drug delivery. Soft matter nanocarriers which are considered as potential delivery system like liposomes, micelles, nanoparticles based on biological (chitosan, gelatin) or synthetic materials (polyglycerol, polylacticoglycolid) can show good biocompatibility, can deliver a broad variety of molecules, can easily be prepared and surface modified, and allow stimuli-responsive payload releases.
The aim of this review is to highlight and converse about technological advances and approaches in the field of soft matter nanocarriers for the dermal delivery of therapeutic proteins and peptides, and to provide perception into the advantages, limitations, and practicability of recent developments. We focus on nasal, dermal, pulmonary and ocular routes of biomacromolecule administration which having several advantages over classic injections.
Polymeric NPs as Carriers for Proteins and Peptides
Polymeric NPs are solid colloidal carriers composed of natural, synthetic or semi-synthetic polymers with size ranging from 10 to 1000 nm [Bergers,2005, Yao,2011]. These solid colloidal carriers are usually classified as either nanospheres or nanocapsules. In nanospheres, drug is dispersed in polymeric matrix whereas nanocapsules are reservoir system in which drug is confined within a polymeric shell. Both polymeric nanospheres and nanocapsules have been explored for the delivery of protein and peptide therapeutics. Properties of polymeric NPs are significantly affected by the method of preparation and the nature of polymers. Chitosan (CS), alginate and gelatin are example of common natural polymers.
These polymers are appropriately present in nature and have been extensively applied in peptides delivery and oral proteins. Among natural polymers, Chitosan has shown most interesting potential, attributed to its better solubility at the intestinal pH, permeation enhancement and improving mucoadhesivness. In the small intestine, CS NPs can adhere and infiltrate into mucus layer and open the tight junctions between contiguous epithelial cells [4]. Moreover, pH-sensitive CS NPs can disintegrate and release the encapsulated drugs which then penetrate through the opened paracellular pathway. Examples of synthetic polymers investigated for proteins and peptides delivery are poly(lactic acid) (PLA), polyacrylic acid (PAA) poly(DL-lactide co-glycolide) (PLGA), polycaprolactone (PCL), polyalkylcyanoacrylate and poly(methyl methacrylates) [Banga,1993,Bj¨orn,2015,Chellgren,2006]. Among polyesters, synthetic polymers alone or in combination are the most frequently and most relevant studied for protein delivery. Contrary to natural polymers, synthetic polymers enable adjustable controlled drug release for a period of several days to weeks.
Proteins could be encapsulated, adsorbed or chemically linked to the surface of polymeric NPs. Protein incorporation in polymeric NPs can be achieved by various methods. Natural polymers are generally highly sensitive to processing conditions. Therefore, NPs with natural polymers are generated using mild techniques including coacervation, polyelectrolyte complexation and ionic gelation [Chereddy,2014,Lappin-Scott,1995,Alonso,2006]. NPs composed of synthetic polymers are normally prepared by more extensive techniques such as emulsification-solvent evaporation, interfacial polymerization, emulsification– polymerization, nanoprecipitation, salting out, supercritical fluids and emulsification solvent diffusion [Garinot,2006, Eley,2007, Kumar,2011, Takeuchi,2000, Hancock,2006, Perera,2011, Swogger,2008].
Skin physiology and challenges
Dermal and Transdermal applications are important drug delivery Route. A huge amount of topical formulations are on the market and around 33% of drugs in clinical trials focus on topical applications. Transdermal and Dermal application allows easy drug administration with the possibility to control its release and to avoid the first-pass metabolism.
The skin is a complicated structure that protects the human body from environmental factors and controls essential processes. Dermal absorption is inhibit by the stratum corneum when applying a drug topically. Stratum corneum is the main skin barrier, which needs to be control to reach its desire target site, which can either be in systemic circulation or transdermal. The stratum corneum consists of close and flattened corneocytes that are inserted in a highly lipophilic lipid matrix consisting of ceramides, cholesterol, and fatty acids (fig.2). Beneath the SC, the viable epidermis is made up of viable keratinocytes which are organized in three sub-structures: the stratum basale, stratum spinosum, stratum granulosum. The dermis, which contains blood vessels as well as nerve fibers, consists of a thick network formed by proteins, collagen, and elastic fibers and gives structural base for the viable epidermis (fig.2). Due to the distinctive properties of the stratum corneum SC, efficient skin absorption is only obtained with rather small drugs (molecular weight ≤ 500 Da, total cutoff 800 Da) which also exhibit a moderate lipophilicity (log P = 1–3). Because of high molecular weight (between 300 and 1,000,000 Da) and amphoteric properties of biomacromolecules, they are almost completely restricted from transdermal penetration.
However, topical application of biomacromolecules seems promising for particular indications. When administered systemically, peptides and proteins reveal a short half-life which makes repeated injections unpreventable. Topical applications could extend their half- life and permit for a controlled release from the formulations which helps to maintain unchanging blood concentrations. Based on this examination, the dermaland transdermal delivery of biomacromolecules like calcitonin and insulin has frequently been pursued. Moreover, for the treatment of severe skin diseases using topically applied biomacromolecules is new but highly interesting approach. This is of particular interest for a replacement therapy of proteins that are deficient in the skin due to genetic mutations and, thus, cause severe skin diseases.
In order to achieve systematic protein and peptide delivery into the skin, various attempts have been employed that often involve invasive methods microporation or so no phoresis. Also, these methods mostly lack broad applicability. As long as penny plain biomacromolecules are restricted from suitable formulations, dermal penetration, and carrier system are needed and soft matter nanocarriers might have the capacity to meet the demands. In particular liposomal formulations, biphasic vesicles, were employed quite interesting results which will be discussed hereinafter.
Challenges for dermal delivery of antimicrobials proteins and peptides:
We have previously reviewed the discrete mechanism for loss of activity of proteins and peptides along with role of nanotechnology in addressing the various issues in delivery including conformational stability and defence of biological activity, overcoming immunogenic and biologic barriers to improve bioavailability etc [Rades,2011]. Dermal route of delivery is a non-invasive and convenient route for treatment of several types of bacterial, fungal infections, inflammation, wounds and other skin pathophysiological conditions. Due to the non-invasiveness, ease of accessibility, delivery of the macromolecule therapeutics like protein and peptide by transdermal and dermal transdermal delivery makes this route most promising and interesting [Park,2014]. In treatment of the skin infections, Antimicrobial protein and peptides are favorable candidates for delivery. Furthermore, dermal route of the protein and peptides is limited due to many barriers.
Skin itself is the most prominent barrier for the delivery of the protein and peptides. Hydrophilic drugs and charged molecules are difficult to penetrate the stratum corneum (SC) of the skin layer. Due to lipophilic nature of the skin layer, hydrophilic drugs cannot penetrate. To control the barrier of the SC, many approaches have been broadly studied which includes the physical and chemical approaches. Physical approaches cover microneedle injection, iontophoresis, radiofrequency ablation, electroporation, thermal and laser ablation, while chemical approaches cover the changes of the peptide and proteins, use of chemical inducers and increasing the skin penetration by developing suitable formulations like use of prodrugs, use of encapsulation technologies, etc [Park,2005, Mahapatro,2011]. Furthermore, the use of chemical methods for enhancing permeation across stratum corneum(SC) possesses toxicity issues and physical methods are expensive and inconvenient. Some of the AMPs act as skin penetration increase itself by the interrupting the stratum corneum(SC) layer. Pore forming peptide, Magainin, have been reported as a permeability inducer alone and in combination with the other chemical enhancers like N-lauroyl sarcosine and ethanol. The research was carried out to assess the influence of structural changes on penetrability and it was concluded that retention of native structural conformation is important for favoring penetration across the skin [Tozuka,2010].
It is important for the protein and peptides to penetrate the deeper layers of the skin for their therapeutic effect and better efficiency. The second challenge for protein and peptide drugs when they are delivered transdermally or dermally, is to prevent their enzymatic degradation [Mukherjee,2008]. Nevertheless, the proteolytic activity of the enzymes in the skin is less compared to other routes, but the universal presence of enzymes like exo and endopeptidases cleave the protein and peptide terminal residue leading to loss of activity [Vibhuti,2012]. Use of the protease inhibitors with the protein and peptide delivery is pursue the alternative approach to control the challenge.
Chances of obtain immune reaction to the administered peptide was observed in research involving use of short chain peptide compatible to histoneH3 region. The peptide was bind to liposomes in which the surface bound fraction of the peptide contributed for immunogenicity [Amrutiya,2015]. A similar discovering was observed for polyamide (peptide) nucleic acids (TAR)-penetration conjugate administered in mice due to peptide conjugation [Sandreschi,2015]. Thus, this is supreme consideration should be given for encapsulation of protein and peptides and selection of formulation components.
Nanocarrier systems for dermal delivery of protein and peptides
Liposomes
Topical vaccination is potential field of application and became of increasing interest during the last few years. Administration via intramuscular or subcutaneous injections, classic vaccination is accompanied by infection risks, pain or anxiety at the injection site. Thus, the development of non-invasive and alternative routes is explored, and dermal vaccination is of particular interest as the skin contains a complicated network of immune dendritic cells and Langerhans and is responsive to antigens.
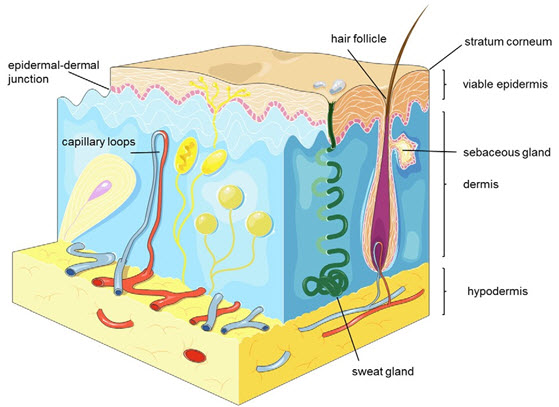
Figure 2. Schematic depiction of the different skin layers, appendages and vessels.
Two different types of flexible liposomes made up of cholesterol and phospholipids S 100 for efficient transcutaneous delivery of the model antigen ovalbumin (molecular weight approx. 45kDa). After 30 min. of topical application, particle collection in the hair follicles and sweat ducts was shown, but then after no distribution of the liposomes in the skin was observed. No major changes in the immune response of barrier disrupted skin were observed, and in combination with the adjuvant imiquimod a equivalent immunization was observed. For topical vaccination, hair follicles are of particular interest which can facilitate the absorption of large molecules like antigens. Here, the nanocarrier size is an important factor especially large molecules permeate beyond the infundibulum towards the abundant perifollicular Langerhans cells. Thus, many groups studied the administration of antigens for transcutaneous immunization. An adjuvant, furthermore, seemed to be important for efficient immune responses in non-invasive hair follicle mediated immunization.
The particular application of biomacromolecules can also serve other rationale, for example the treatment of cationic lipopeptide, severe skin conditions, developed sterically stabilized liposomes, and encapsulated recombinant human transglutaminase 1 (molecular mass 92 kDa) in order to address the under lying cause of autosomal recessive congenital ichthyosis. The unilamellar follicle was made up of poly (ethylene glycol)-2000-dipalmitoyl-phosphatidylethanolamine and phosphatidylcholineand were about 200 nm in size. To show the effectiveness of the concept in vivo, a skin-humanized mouse model was used. Following the application of the liposomal formulations, a substantial improvement of the phenotype was seen already. According to the study of Stout et al. demonstrated the strong engineering of a cell penetrating peptide—filaggrin complex and its proper permeation into epidermal tissue after topical application as well as the restoration of the pathological phenotype in filaggrin deficient flakytail mice. These two approaches provided the proof of concept for a topical protein substitution therapy and described promising results with potential applicability in the future
Biphasic vesicles
Biphasic vesicles built up from different nanoscale components might be complementary for the topical delivery of drugs and antigens. Biphasic vesicles are lipid-based delivery systems that combine the structures of liposomes and emulsions and these vesicles have oily, aqueous, and micellar compartments which are enclosed by concentric phospholipid bilayers and allow a flexible composition for optimized encapsulation. Biphasic vesicles were researched for the topical delivery of, e.g., albumin (60 kDa), insulin (6 kDa), interferon alpha (19 kDa), or antigens like hen egg lysozyme (14 kDa), or staphylococcal enterotoxin (28 kDa) , leukotoxin (100 kDa),
An interesting application was discover by King et al. who inspect the efficacy of lipid- based biphasic vesicles (Biphasix™) for dermal insulin delivery. The biphasic vesicles consisted of a complicated mixture of propylene glycol, soya phosphatidyl choline, cholesterol and linoleamidopropyl-PG-dimonium chloride phosphate. Insulin-loaded vesicles were finally incorporated into a patch which applied onto diabetic Sprague Dawley rats. A pharmacologically relevant reduce in blood glucose was observed for about 52 h, and the serum insulin levels did not differ from the effects achieved by subcutaneous insulin injection. Further studies substantiated these findings. Furthermore, dermally applied insulin is less effective in glucose lowering compared to, pulmonary, oral or buccal application (oral: 20–50%, pulmonary: 45–80%, buccal: 15–75% versus 25–38% indermal)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Microemulsions
In addition to proteins, peptide-based therapeutics is interesting and important biopharmaceutical products because of their higher affinity and target specificity, simple manufacturing process. In general, because of their molecular size, peptides give better issue permeation properties and are less immunogenic than proteins. In this course,
Goebel et al. targeted for advanced skin penetration of the N-acetyl-L- carnosine (molecular weight 268.27 Da) and antioxidative dipeptides carnosine (molecular weight 226.23 Da). The peptides were manufactured in a microemulsion, containing phospholipon 90G, plantacare2000UP, cetiolB, polyglycerol, and purified water. A particular skin permeation improvement was achieved, but a fast and substantial loss of L-carnosine because of biodegradation and in stabilities was also observed, which is a common problem for biomacromolecules. This research highlights the significance of an adequate and protective drug carrier system for labile compounds.
Polymeric nanoparticles
Polymeric nanoparticles are searched widely as carriers for controlled or sustained release (CR/SR) in drug delivery systems. Polymeric nanoparticles may be either nanospheres or nanocapsules. Two main approaches used for manufacturing of polymeric nanoparticles are the “bottom-up” approach and the “top-down” approach. In the top-down approach a diffusion of preformed polymers produces polymeric nanoparticles, whereas in the bottom-up approach polymerization of monomers leads to the manufacturing of polymeric nanoparticles.
Different methods for producing polymeric nanoparticles are illustrated in Fig. This figure has been taken from Sabliov and Astete (2008), Rao and Geckeler (2011), and Ezhilarasi et al. (2013) changed accordingly.
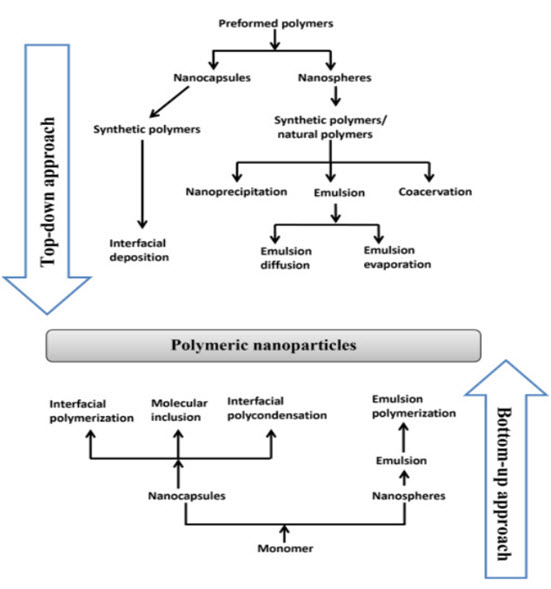
Figure 3. Different methods for producing polymeric nanoparticles
The choice of determine an particular method for formation of polymeric nanoparticles depends on various factors like types of solvents and polymers used in, like particle size, synthesis, area of application and so on. The “top-down” method for formation of polymeric nanoparticles involve solvent emulsification–diffusion (emulsion diffusion method), nanoprecipitation (solvent displacement method)solvent emulsification–evaporation (emulsion evaporation method), coacervation, interfacial polymerization ,Emulsion polymerization, interfacial poly condensation, and molecular inclusion use monomers as their starting point and come under the “bottom-up” process to produce polymeric nanoparticles.
Both the methods, top-down and bottom-up use synthetic polymers/monomers such as poly(butyl cyanoacrylate), poly(ethyl cyanoacrylate), poly(d, l-lactide-co-glycolide), poly(isohexyl cyanoacrylate), poly(isobutyl cyanoacrylate); organic solvents such as dichloromethane and ethyl acetate, acetonitrile, acetone, benzyl alcohol, cyclohexane; and stabilizers like poly(vinyl alcohol) and didecyldimethylammonium bromide; so on that are toxic. Recently the scientific amity has been trying to find replacement for synthetic polymers by using natural polymers with less toxic solvents.
This investigation does not give contrast information about all the methods and synthesis protocols used in preparing polymeric nanoparticles. There are brilliant reviews available in the literature that gives information on the different formulation methods of polymeric nanoparticles synthesis
Antimicrobial peptide delivery: for the treatment of burn and wounds
AMPs, which are novel, multifunctional agents with diversity in amino acid sequences in their structure, are emerging as new therapeutics. Over the past two decades, AMPs have been discovered from natural sources, in other words, plants, microbes, tissue extracts, insects etc. Moreover, many AMPs also have been synthetically designed in recent years. Basically, Antimicrobial peptide display the net positive charge that may allow the electrostatic attraction toward the cell membranes (negatively charged) of the microbes [Poindexter,2005, Werb,1988]. As discussed earlier, Antimicrobial peptide may be classified as α-helix, β-sheet, linear and looped peptides on the basis of secondary backbone in their structures [Banga,1993]. Additionally, their amphipathic and hydrophobicity nature offers cell penetrating and conformational flexibility properties.
AMPs mainly act by two mechanisms: pore dependent and independent. Most AMPs have the pore-dependent mechanism in which it may interact with the cell membranes and alter the membrane integrity, ultimately leading to permeabilization/disruption of membranes. Different models (Figure 4) have been proposed to describe this mechanism of action of AMPs, in other words, Toroidal pore model, Barrel–Stave model, Carpet model, Membrane depolarization, Electroporation, etc [Maria,2010].
Role of AMPs in burns & wounds
Chronic infected burns and wounds may result from diabetic foot and venous leg ulcers, infections at the surgical site, and may frequently suffer from persistent inflammation and are more often infected with many microorganisms [Davidson,2002]. Moreover, these microorganisms can play an important role in the recurrence and resistance of the infectious burns and wounds. They mostly exist within the biofilms, which is a slimy, hydrated polymeric matrix embedded with bacteria attached to solid surface of skin, and may show an altered phenotype with respect to their growth rate and gene transcription too [Shai,2002, Hammond,2009].
With the recent dilemma of globally impaired resistance specially with the multiresistant infectious microbes (i.e., K. pneumoniae, S. aureus, P. aeruginosa, Enterococcus faecium, Acinetobacter baumannii, etc.), and also the lack of availability of new antimicrobials, efforts are being made to develop and discover AMPs with broad-spectrum antimicrobial activity to combat these serious infections associated with burns and wounds as novel therapeutic agents.
In a wound, TGF-α and IGF-1 are the human cathelicidin, LL-37 stimulators. Each of these plays a significant role in wound healing by means of stimulating fibroblasts and epidermal cells to form granulation tissue that can promote angiogenesis. In addition, its chemo attractant properties also lead to the stimulation of receptor-mediated proliferation of the endothelial cells [Silva,2015, Clark,1999]. Furthermore, LL-37 also has properties for controlling the inflammation via a reduce in the production of inflammatory mediators induced by bacteria [Sinsuebpol,2013], confirming the role of LL-37 as a modulator of innate immunity. While in the case of human-β-defensins, burned skin, which are 2–6 kDa cationic AMPs, and a family of mammalian defensins, are expressed prominently. However, the high concentrations of LL -37 are also detected in sweat ducts of the epithelium. Authors also concluded that cells in subdermal and dermal areas of burned skin yield human-β-defensins subsequently to preserve a barrier to counter infection [Steinstraesser,2006, Steinstraesser,2002]. Another synthetic peptide (PXL150) showed an antimicrobial effect in P. aeruginosa infected mice, which is the common bacterial species found in burn wound infections, and therefore may be another novel therapeutic agent [Sonaje,2012]. Additionally, antimicrobial effects of Novispirin G10 and β-sheet forming peptide IK8L (synthetic peptides) were widely effective against clinical isolates of human burn wounds as well as in in vitro infected burn wound models colonized with P. aeruginosa [Bennett,2010].
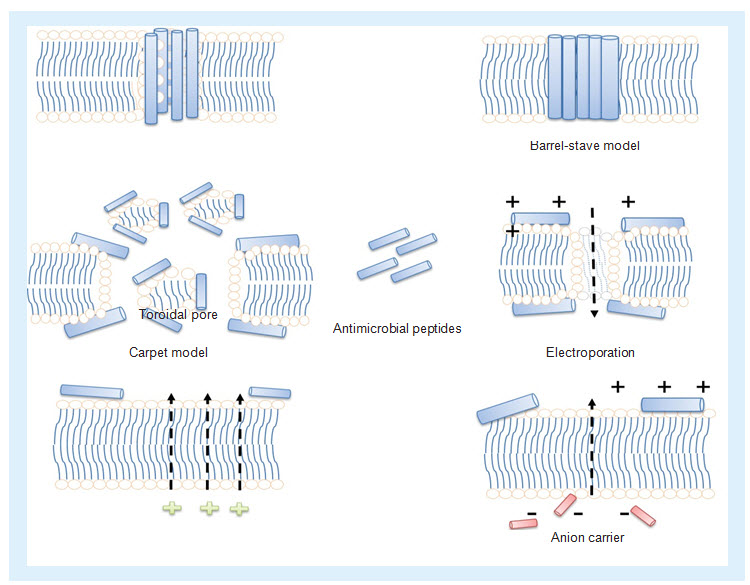
Figure 4. Different mechanism of actions of antimicrobial peptides
(i) Toroidal pore: antimicrobial peptides (AMPs) bind to the membrane and force lipids to fold inward to form a channel lined by lipid headgroups and AMPs at the membrane interface. (ii) Barrel-Stave model: AMPs fix themselves perpendicular to and are proposed to span the lipid bilayer. (iii) Carpet model: AMPs forms a carpet that is able to induce weakness within the lipid bilayer by means of destroying electrostatics. (iv) Electroporation: the binding of AMPs may induce pore formation through an electroporation in addition to the existence of mechanical stress. (v) Membrane depolarization: AMPs having the ability to depolarize the lipid bilayer. (vi) Anion carrier: pore formation of AMPs in the lipid bilayer by means of anion carrier.
A promising candidate LLKKK18 conjugated with dextrin exhibits excellent healing properties. Authors also demonstrated the angiogenic action of LLKKK18 in C57-BL/6 mice, which was performed by immunolabeling for a α-smooth muscle actin in the tissue sections, a protein existing in the surrounding microvessels of the smooth muscle cells [Yount,2003, Shen,2001].
Clinical outcome
The dermal delivery of biomacromolecules using soft matter nanocarriers is still rarely labeled. In contrast, skin barrier modifying methods like sonophoresis and microporation were investigated more rapidly.
Elimination and biodistribution of loaded biomacromolecules affected due to nanoparticles properties. The nanocarriers should be relatively small for the size to regulate the penetration depth as well as moderate ionization and amphiphilic properties facilitate in- tractions between the carrier system or skin surface. It is also significant to mention potential risks considering specific dermal protein application can activate immunologic responses. These events additionally grow in diseased skin which is characterized by an impaired barrier function. Nowadays, some publications relate the skin absorption of nanoparticles while others did not validate these findings even in skin disease. One problem is mainly different in particle size, components, surface properties is very large range of fabricated nanoparticles. Thus, the nanoparticles' fate required to be estimate individually; it is not possible the final and common prediction. Currently investigated in the course of topical vaccination are peptide delivery and dermal protein. Just recently at least two clinical trials trained for transcutaneous vaccine delivery via the hair follicles were initiated or conducted. Furthermore, the evidence of concept for a local protein substitution therapy, which may open a new treatment option for severe skin diseases. These evidence of concept for a local protein substitution therapy provided by recent researches. Advance investigations are crucial to allow for conclusions about the practicability of this approach because these studies, however, are at a very early stage.
REFERENCES:
1. Banga, A.K., Chien, Y.W. (1993); Dermal absorption of peptides and proteins. Biol. BarriersProteinDeliv., 4(77); 179–197.
2. Bergers, G., Song. S.(2005); The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol; 7(4); 452–464.
3. Bi, L., Yang, L., Narsimhan, G., Bhunia, A.K., Yao, Y(2011); Designing carbohydrate nanoparticles for prolonged efficacy of antimicrobial peptide; J.Control. Rel; 150(2); 150–156.
4. Bilati, U., Allemann, E., Doelker, E.(2005); Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues; AAPS PharmSci Tech; 6(4); E594–604.
5. Bj¨orn, C., Noppa, L., Salomonsson, E.N.et al. (2015); Efficacy and safety profile of the novel antimicrobial peptide PXL150 in a mouse model of infected burn wounds. Int. J. Antimicrob. Agents; 45(5); 519–524.
6. Chellgren, B.W., Creamer, T.P. (2006); Side-chain entropy effects on protein secondary structure formationProteins 62(2); 411–420.
7. Chereddy, K.K., Her, C-H., Comune, M.et.Al. (2014); PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J.Control. Rel; 194; 138–147.
8. Costerton, J.W., Lewandowski, Z., Caldwell, D.E., Korber, D.R., Lappin-Scott, H.M. (1995) ; Microbial biofilms. Annu. Rev. Microbiol; 49(1); 711–745.
9. Cuña, M., Alonso-Sandel, M., Remuñán-López, C., Pivel, J.P., Alonso-Lebrero, J.L., Alonso, M.J.(2006); Development of phosphorylated glucomannan-coated chitosan nanoparticles as nanocarriers for protein delivery. J Nanosci Nanotechnol; 6(9-10); 2887–95.
10. Des, Rieux. A., Fievez, V., Garinot, M., Schneider, Y.J., Préat, V. (2006); Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 116(1); 1–27.
11. Eley, J.G., Mathew, P.(2007); Preparation and release characteristics of insulin and insulin-like growth factor-one from polymer nanoparticles; J Microencapsul; 24(3), 225–34.
12. Foldvari, M., Badea, I., Kumar, P.et. al. (2011); Biphasic vesicles for topical delivery of interferon alpha in human volunteers and treatment ofpatients with human papillomavirus infections. Curr. Drug Deliv; 8(3); 307–319.
13. Ghahary, A., Tredget, E.E., Shen, Q., Kilani, R.T., Scott, P.G., Takeuchi, M.(2000); Liposome associated interferon-alpha-2b functions as ananti-fibrogenic factor in dermal wounds in the guinea pig; Mol.Cell Biochem; 208(1); 129–137.
14. Hancock, R.E., Sahl, H.G.(2006); Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies; Nat. Biotechnol; 24(12); 1551.
15. Iqbal, J., Vigl, C., Moser, G., Gasteiger, M., Perera, G., Bernkop-Schnürch, A.(2011); Development and in vivo evaluation of a new oral nanoparticulate dosage form for leuprolide based on polyacrylic acid; Drug Deliv; 18(6); 432–40.
16. James, G.A., Swogger, E., Wolcott, R.et.Al. (2008); Biofilms in chronic wounds. Wound Repair Regen., 16(1); 37–44.
17. Kafka, A.P., McLeod, B.J., Rades, T., McDowell, A.(2011); Release and bioactivity of PACA nanoparticles containing D-Lys(6)-GnRH for brushtail possum fertility control. J Control Release; 149(3); 307–13.
18. Lee, Y.W., Park, M.K., Shin, J.R., Lim, K.J., Cho, J.H., Kim, S.C. (2014); Efficacy of the designer antimicrobial peptide SHAP1 in wound healing andwound infection; Amino Acids, 46(10); 2333–2343.
19. Li, H., An, J.H., Park, J.S., Han, K. (2005); Multivesicular liposomes for oral delivery of recombinant human epidermal growth factor; Arch. Pharm Res., 28(8); 988–994.
20. Mahapatro, A., Singh, D.K. (2011); Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines; J Nanobiotechnol; 9:55.
21. Makhlof, A., Werle, M., Tozuka, Y., Takeuchi, H.(2010); Nanoparticles of glycol chitosan and its thiolated derivative significantly improved the pulmonary delivery of calcitonin. Int J Pharm; 397(1-2); 92–5.
22. Mukherjee, B., Santra, K., Pattnaik, G., Ghosh, S.(2008); Preparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers; Int J Nanomedicine; 3(4); 487–96.
23. Patel, A., Cholkar, K., Vibhuti, A., Mitra, A.K. (2012); Ocular drug delivery systems: An overview. World J Pharmacol, 2(2); 47–64.
24. Patil, S., Vhora, I., Amrutiya, J., Lalani, R., Misra, A. (2015); Role of nanotechnology in delivery of protein and peptide drugs. Curr. Pharm. Des.; 21(29); 4155–4173.
25. Piras, A.M., Maisetta, G., Sandreschi, S.et. al. (2015); Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-termantibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis; Front. Microbiol; 6, 372.
26. Poindexter, B.J. (2005); Immunofluorescence deconvolution microscopy and image reconstruction of human defensins in normal and burnedskin; J. Burns Wounds; 4, e7.
27. Rappolee, D.A., Mark, D., Banda, M.J., Werb, Z. (1988); Wound macrophags express TGF-a and other growth factors in vivo: analysis by mRNA phenotyping; Science, 241(4866); 708–12.
28. Santander-Ortega., Manuel, J. Csaba, N., González, L., Bastos-González, D., Ortega-Vinuesa, J.L., Alonso, Maria, J.(2010); Protein-loaded PLGA–PEO blend nanoparticles: encapsulation, release and degradation characteristics. Colloid Polym Sci; 288(2); 141–150.
29. Scott, M.G., Davidson, D.J., Gold, M.R.,Bowdish, D., Hancock, R.E.(2002); The human antimicrobial peptide LL-37 is multifunctional modulatorof innate immune responses. J. Immunol; 169(7); 3883–3891.
30. Shai, Y.(2002); Mode of action of membrane active antimicrobial peptides. Biopolymers; 66(4); 236–248.
31. Shukla, A., Stephanopoulos, G., Hammond, P.(2009); Antimicrobial peptide delivery from degradable polymer thin films. 3-5 A.
32. Silva, J.P., Dhall, S., Garcia, M.etal. (2015); Improved burn wound healing by the antimicrobial peptide LLKKK18 released from conjugates withdextrin embedded in a carbopol gel. Acta Biomater; 26; 249–262.
33. Singer, A.J., Clark, R.A.(1999); Cutaneous wound healing. N. Engl. J. Med., 341(10); 738–746.
34. Sinsuebpol, C., Chatchawalsaisin, J., Kulvanich, P.(2013); Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery; Drug Des DevelTher; 7:861–73.
35. Steinstraesser, L., Ring, A., Bals, R., Steinau, H-U., Langer, S. (Steinstraesser,2006). The human host defense peptide LL37/hCAP accelerates angiogenesis inPEGT/PBT biopolymers; Ann. Plast. Surg. 56(1); 93–98.
36. Steinstraesser, L., Tack, B.F., Waring, A.J.et al. (2002); Activity of novispirin G10 against Pseudomonas aeruginosain vitro and in infected burns.Antimicrob. Agents Chemother., 46(6); 1837–1844.
37. Sung, H.W., Sonaje, K., Liao, Z.X., Hsu, L.W., Chuang, E.Y.(2012); pH-responsive nanoparticles shelled with chitosan for oral delivery of insulin: from mechanism to therapeutic applications; AccChem Res; 45(4); 619–29.
38. Wolcott, R., Rhoads, D., Bennett, M.et. al.(2010); Chronic wounds and the medical biofilm paradigm. J. Wound Care 19(2); 45–53.
39. Yadav, S.C., Kumari, A., Yadav, R.(2011); Development of peptide and protein nanotherapeutics by nanoencapsulation and nanobioconjugation, Peptides; 32(1); 173–87.
40. Yeaman, M.R., Yount, N.Y.(2003); Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev; 55(1); 27–55.
41. Zhang, Q., Shen, Z., Nagai, T.(2001); Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int J Pharm; 218(1-2); 75–80.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









