 About Authors:
About Authors:
Marvinkumar I. Patel*, Ravi R. Patel
Nootan Pharmacy College,
Visnagar,
Gujarat, India
*patel.marvin62@gmail.com
Abstract:
Dendrimer chemistry was first introduced in 1978 by Fritz Vogtle and coworkers. He synthesized the first “cascade molecules”, today known as dendritic molecules. The dendrimer architecture permits control over properties such as shape, size, density, polarity, reactivity and solubility. Dendrimer density functions and starburst limits can be easily modeled mathematically. Dendrimers have stimulated wide interest in the field of chemistry and biology, especially in applications like drug delivery, gene therapy and chemotherapy. A treatment of Cancer mainly focused on the targeting the active drug molecule at the site without affecting the neighbour cells and dendrimers have this property which is useful in diagnosis and treatment purpose which is a new hope in this area of Cancer treatment.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1284
INTRODUCTION
The word “dendrimer” originated from two words, the Greek word dendron, meaning tree, and meros, meaning part. Dendrimer chemistry was first introduced in 1978 by Fritz Vogtle and coworkers. He synthesized the first “cascade molecules”, today known as dendritic molecules. In 1985, Donald A. Tomalia, working in the field of polymer chemistry, synthesized the first family of dendrimers.1 These contributions to the field have paved the way for continuing research in this promising area. The term ‘dendrimer’ refers only to an architectural motif and not a particular compound. To date greater than 160 various polymers with dendritic structures are reported in the literatures. The surface groups of dendrimers are amenable to modification and can be tailored for specific applications. The dendrimer architecture therefore permits control over properties such as shape, size, density, polarity, reactivity and solubility. They are produced in an iterative sequence of reaction steps, in which each reaction results in a new so called generation. Dendrimer density functions and starburst limits can be easily modeled mathematically. These features are related to core multiplicity, the branching multiplicity of the monomer units, and the branch lengths, as well as the core and branch volumes.2 Due to their multivalent and monodisperse character, dendrimers have stimulated wide interest in the field of chemistry and biology, especially in applications like drug delivery, gene therapy and chemotherapy.
DENDRIMERS IN CANCER DIAGNOSIS AND TREATMENT
Dendrimers Have Attractive Properties For Cancer Treatment
Cancer epitomizes the challenges faced during drug delivery: an anticancer drug must be able to seek out subtle changes that distinguish a transformed cell from the other 200 or so types of healthy cells found in the body and then provide a sufficiently high dose of a toxic agent to selectively kill the cell while not harming its healthy neighbors. Therefore, even though dendrimers can be endowed with many favorable properties for drug delivery, an ultimate challenge – ergo, a ‘‘real-world’’ test of these versatile nano-devices will be whether they can successfully meet the formidable tasks of diagnosing and treating of malignant disease.
DENDRIMER-SIZED PARTICLES PASSIVELY ACCUMULATE AT THE SITES OF TUMORS
To begin the discussion of properties that make dendrimers attractive vehicles for cancer treatment, we revisit the concept that encapsulation or covalent linkage of small molecule drug candidates to a dendrimer enhances the pharmacological properties of the drug. In cancer chemotherapy, these desirable size-based features are reinforced by the enhanced permeability and retention (EPR) effect that improves the delivery of macromolecules to tumors. The EPR effect is based on unique pathophysiological features of a solid tumor, such as extensive angiogenesis resulting in hyper-vascularization, limited lymphatic drainage, and increased permeability to lipids and macromolecules. These features, which help ensure adequate nutrient supply to meet the metabolic requirements of rapidly growing tumors,5-6 can be turned to the tumor’s disadvantage by the use of nano-sized therapeutic agents. The EPR effect was discovered when selective accumulation of the SMANCS conjugate (styrene-maleic anhydride-neocarzinostatin) was observed at the site of tumors while similar accumulation was not seen with neocarzinostatin alone.7-8 The EPR response was subsequently demonstrated for similarly-sized liposomes, thereby establishing that this effect was largely a function of particle size and did not solely depend on the chemical or biophysical properties of the macromolecule. Specifically, in one study optimal tumor delivery occurred for liposomes having a size distribution between 70 and 200 nm in diameter.9 An independent study showed efficacy for liposomes loaded with daunorubicin in the same size range; specifically, those@142 nm in diameter exhibited an inhibitory effect against Yoshida sarcoma whereas smaller (@57–58 nm) and larger (@272 nm) liposomes had weaker or no effect.10 Over time, cautionary notes were raised that tempered initial enthusiasm for exploiting the EPR effect for cancer treatment. For example, the porosity of the vasculature in tumors can be highly variable even with a single vessel that can be leaky to one size of particle in one region but not in another.11 Experimentally addressing this issue was complicated by the size polydispersity of traditional nanoparticles used to exploit the EPR effect, which were typically either lipids or conventional polymers that rendered a significant proportion of intended drug inactive. Fortunately this issue – the ability to match exact and uniform sizes needed to target an individual tumor is highly tractable with dendrimers because selection of an exactly-sized entity is possible (Table 1) compared with the large size distributions that plague liposome and most polymeric materials.12
[adsense:468x15:2204050025]
Table 1.Generation by generation specifications for PAMAM Starburst dendrimers.
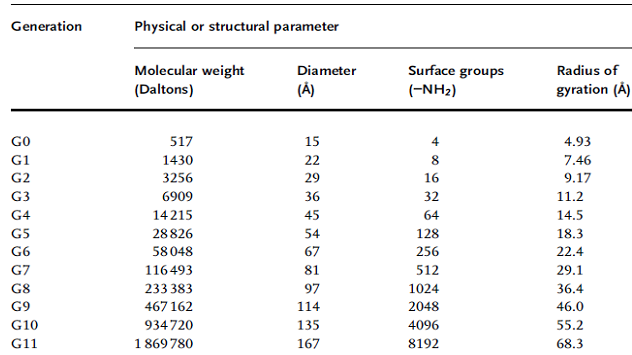
The ability to construct monodisperse populations of dendrimers in the size range needed to exploit the EPR effect is an encouraging step towards the passive exploitation of tumor properties. Once the basic issue of size was resolved, however, secondary challenges (and opportunities) arose from observations that the chemical properties of the nano-sized particle can play significant roles in modulating the EPR effect. By way of a specific example, ‘‘conventional’’ polymeric materials showed efficacy at a smaller size range, occurring at 60 nm for both water soluble and hydrogel forms of poly (vinyl alcohol) (PVA)13, whereas almost identically-sized 57 nm egg phosphatidylcholine (EPC)-liposomes were ineffective. As reported above, liposomes about twice this size showed maximal efficacy, so it was not unexpected that the EPC-liposomes were ineffective. Interestingly, however, hydrogenated egg phosphatidylcholine (HEPC)-liposomes in this size range (specifically, 58 nm) were active, illustrating that the exact chemical properties of the material is a critical design parameter. In this respect, the many options for dendrimer ‘‘building blocks’’, as well as the ability to further tune surface properties provide many opportunities to endow dendrimers with favorable ‘‘Passive” properties for tumor targeting.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MULTIFUNCTIONAL DENDRIMERS CAN SELECTIVELY TARGET BIOMARKERS FOUND ON CANCER CELLS
Methods for Targeting Specific Biomarkers of Cancer
As discussed above, dendrimers can achieve passive EPR-mediated targeting to a tumor simply by control of their size and physicochemical properties. Passive targeting, which localizes the nano-particle in the close vicinity of a cancer cell, can be immediately useful for diagnostic purposes or for the delivery of radioisotopes capable of killing any cell within a defined radius. In general, however, most delivery strategies require that the anticancer agent directly attached to, or be taken up by, the target cell. The ability to append more than one type of functionality to a dendrimer allows the inclusion of ligands intended to bind specifically to cancer cells in the design of a multi-functional drug-delivery nanodevices. Although a wide range of targeting ligands have been considered, including natural biopolymers such as oligopeptides, oligosaccharides, and polysaccharides such as hyaluronic acid, or polyunsaturated fatty acids,14 discussion here is limited to folate, which is an exemplary small molecule tumor-targeting agent, as well as monoclonal antibodies directed against tumor associated antigens (TAAs).
TARGETING BY FOLATE, A SMALL MOLECULE LIGAND
Folate is an attractive small molecule for use as a tumor targeting ligand because the membrane-bound folate receptor (FR) is over expressed on a wide range of human cancers, including those originating in ovary, lung, breast, endometrium, kidney and brain.15 As a small molecule, it is presumed to be non-immunogenic, it has good solubility, binds to its receptor with high affinity when conjugated to a wide array of conjugates, including protein toxins, radioactive imaging agents, MRI contrast agents, liposomes, gene transfer vectors, antisense oligonucleotides, ribozymes, antibodies and even activated T-cells.16-17 Upon binding to the folate receptor, folate-conjugated drug conjugates are shuttled into the cell via an endocytic mechanism, resulting in major enhancements in cancer cell specificity and selectivity over their non-targeted formulation counterparts. Recently, folate has been enlisted in an innovative dendrimer-based targeting schemes.18
TARGETING BY MONOCLONAL ANTIBODIES
Of the many strategies devised to selectively direct drugs to cancer cells, perhaps the most elegant (and demanding!) is the use of monoclonal antibodies that recognize and selectively bind to tumor associated antigens (TAAs).19-22 TAA-targeting monoclonal antibodies have been exploited as delivery agents for conjugated ‘‘payloads’’ such as small molecule drugs and prodrugs, radioisotopes, and cytokines.23-24 The field of ‘‘immunotherapy’’ envisioned almost a hundred years ago, and given renewed impetus a quarter century ago by the development of monoclonal antibody technologies, has nonetheless progressed erratically over the past two decades as many pitfalls have been encountered. Current prospects remain mixed but hopeful; optimistically, progress marked by commercial interest with companies providing their immunotherapeutic drug candidates with flashy trademarked names, such as ‘‘Armed AntibodiesTM’’.25 Similarly, the rosy opinion that this field is ‘‘on the verge of clinical fruition’’ has been published recently.26 Perhaps, more realistically, one recent synopsis holds out ‘‘hope’’ for a major clinical impact for this strategy within the next 10 years. Although a detailed discussion of the many pitfalls encountered in immunotherapy efforts is beyond the scope of this chapter, one key issue – readily addressed by dendrimers – is the requirement that an extremely potent cytotoxic drug be used in targeted antibody therapy. This point is illustrated by the fact that the greatest progress in this field has occurred for immunotoxins, which are antibody–toxin chimeric
Molecules that kill cancer cells via binding to a surface antigen, internalization and delivery of the toxin moiety to the cell cytosol. In the cytosol, protein toxins, such as those from diphtheria or pseudomonas, catalytically inhibit a critical cell function and cause cell death.27 The high potency of immunotoxins for killing cancer cells is dramatically illustrated by ricin, where the catalytic activity of this ribosome-inactivating enzyme allows a single immunotoxin conjugate to kill a cell upon successful uptake and trafficking to the site of action.28-29 A drawback of immunotoxins is their significant immunogenicity, which limits repeated use; from a broader perspective, their repeated use is made necessary by difficulties in providing a sufficiently high drug load to eradicate all cancer cells despite the high potency of conjugated toxin. An alternative approach of radio immunotherapy, where high energy radio nuclides are conjugated to TAA-targeting antibodies, also shows promise but suffers from indiscriminate toxicity (the surrounding healthy tissues, as well as off-target tissues, become irradiated in addition to the target cancer cells).30 A third possible approach for immunotherapy, the conjugation of commonly-used small molecule drugs to TAAs, is hindered by the relatively low potency of most low molecular weight therapeutics. To illustrate this point,@10 000 TAAs occur on a typical cancer cell,31 making this number the upper limit for the number of targeting antibodies that can bind to the cell. The widely used anticancer drug cisplatin, to give one example, requires internalization of at least 50 X this level of drug molecules for therapeutic efficacy.
A numerical analysis of the cisplatin example presented above indicates that each tumor-targeting antibody would have to be modified with a large number of small molecules to be effective as an anticancer drug (in this case, roughly 50 cisplatin molecules upon superficial analysis). Modification of an antibody with multiple radioisotopes, toxins, or even small molecules to increase the efficacy of cell killing, however, diminishes or eliminates the inherent specific antigen-binding affinity of an antibody. Therefore, to maximize drug loading while minimizing the deleterious effects on the biological integrity of the host antibody, an attractive approach is to use a linker molecule, such as a dendrimer, that can be highly conjugated (or internally loaded) with drug while modifying only a single site on the surface of the antibody.32 Methodology to covalently attach antibodies to dendrimers that preserve the activity of the antigen–antibody binding site,33-34 e.g., by chemical modification of their carbohydrates and subsequent linkage to PAMAM,35 has opened the door for the inclusion of dendrimers in immunotherapy,36-37 thereby enhancing the future prospects of this chronically ‘‘almost-there’’ strategy.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
DENDRIMERS IN CANCER DIAGNOSIS AND IMAGING
Labeled Dendrimers are Important Research Tools for Biodistribution Studies
The synthetic ability to attach both a tumor-targeting antibody and a potent payload of anticancer drugs to the same dendritic molecule provides a platform for multifunctional
Nano-scale drug delivery devices, Before this technology can be applied in the clinic, however, its safety and efficacy must be demonstrated; towards this end, fluorescently-modified dendritic conjugates have been used extensively to characterize cell targeting, surface binding, uptake and internalization, and even sub-cellular localization.38 The radio labeled counterparts appropriate for animal studies have allowed detailed examination of the Biodistribution of dendrimers. Several radio-isotopes have been conjugated to dendrimers, including 3H,39 14C,40 88Y,41 111In,42 and 125I.43-46 These studies have established that the chemical and physical properties of dendrimers can be tuned to favor distribution to or away from specific organs and, ultimately, to achieve favorable Biodistribution to tumors. The methods used in these experiments, however, typically requiring post-administration dissection of the host animal to allow the analysis of organ sequestration and tissue distribution of the radioisotope, are clearly not applicable to clinical practice. Instead, they have served as an important stepping stone along the path towards non- or minimally-invasive diagnostic procedures, which are proceeding mainly by the development of MRI contrast agents.
STEPS TOWARDS THE CLINICAL REALIZATION OF DENDRIMER-BASED CANCER THERAPIES
The Stage is now set for Dendrimer-based Cancer Therapy
The use of dendrimers for cancer treatment is still in its infancy with few, if any, applications successfully translated to the clinic. Consequently, their use as diagnostic agents constitutes both an important goal in and of itself, and also a valuable ‘‘baby step’’ towards the ultimate goal of curing cancer. As discussed, the process of actual killing cancer cells entails the complicated process of drug uptake followed by release of the drug into the cytoplasm or nucleus and is clearly a more demanding process than cell surface labeling, or even localization to the vicinity of the tumor, sufficient for diagnostic purposes. Nonetheless, in some cases, the transition from imaging to therapy will be closely linked, as evidenced by efforts now underway to combine antibody-targeted MR imaging nanoparticles with the delivery of anti angiogenic genes intended to inhibit the vascularization to the V2 carcinoma model in rabbits.56 Another promising strategy – boron neutron capture therapy – has undergone impressive development over the past decade and is presented next as a successful demonstration of the promise of dendrimer-based cancer therapies.
BORON NEUTRON CAPTURE THERAPY
Cisplatin-based therapies illustrate the need for multiple conjugations of small molecules – estimated at 50 for this platinum drug – to a targeting antibody. While some efforts are underway to use dendrimeric strategies for platinum drug delivery,57 an even more demanding situation, where thousands of ligands are required per targeting antibody, is provided by boron neutron capture therapy (BNCT). Accordingly, BNCT will be discussed here as an illustrative example of how dendrimers can help overcome high hurdles in the development of innovative cancer therapies. As a brief background, BNCT is based on the nuclear reaction that occurs when boron-10, a stable isotope, is irradiated with low energy (a0.025 eV) or thermal neutrons to yield alpha particles and recoiling lithium-7 nuclei. A major requirement for the success of BNCT is the selective delivery of a sufficient number of boron atoms (@109) to individual cancer cells to sustain a lethal 10B (n, alpha)! 7Li capture reaction.58-59 Considering that the maximal number of antigenic sites per tumor cell is in the range of 100 000, and more commonly only 1/10th that level, an a priori calculation suggests that each targeting antibody must be linked to at least 2000, but preferably closer to 5000, boron atoms. Clearly, a single TAA-targeting antibody cannot be directly conjugated at this level and conventional polymers – e.g., polylysine conjugated with @1700 boron derivatives and linked to a targeting antibody – caused the antibody to lose in vivo tumor localizing properties.60 By contrast, when a PAMAM dendrimer was used for polyvalent boron conjugation, the linked antibody maintained immuno-recognition (although in vivo tumor targeting remained problematic because the conjugated dendrimer had a strong propensity to mislocalize in the spleen and liver). Over the decade since these pioneering efforts were first reported, continued progress has been made to solve problems such as off-target tissue localization, which was traced to the size of the dendrimer and presence of a large number of amine groups on the surface of PAMAM, by exploiting the versatility of dendrimer chemistry. In short, the re-design of boronated, anti-body-targeted dendrimers has culminated in the successful treatment of gliomas in the rat and laid the foundation for translation of this technology into clinical tests in the foreseeable future.61
Conclusion:
Dendrimers, chemically-defined entities with tunable biological properties, have advanced over the past two decades to the point where they stand on the cusp of major contributions to the treatment of cancer in a meaningful way. Although, as has been apparent by the many instances cited throughout this chapter where gaps in knowledge still remain and that must be plugged before dendrimers are ready for wide clinical use, their extreme versatility combined with the extensive research efforts now underway are sure to add sophistication to drugs already in use as well as spur the development of entirely new classes of anticancer therapy.
References:
1. Tomalia DA, Baker H, Dewald J, Hall M, Kallos G. New Class of Polymers: Starburst-Dendritic Macromolecules. Polym J. 1985; 17(1):117-132.
2. Matthews O.A, Shipway AN, Stoddart JF. Dendrimers – Branching Out from Curiosities into New Technologies. Prog Polym Sci. 1998; 23:1-56.
3. Heuser LS, Miller FN. Differential macromolecular leakage from the vasculature of tumors. Cancer Res. 1986; 57: 461-464.
4. Maeda H, Wu J, Sawa T, Matsumura Y. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Controlled Release. 2000; 65: 271-284.
5. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986; 46: 6387-6392.
6. Seymour LW. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit Rev Ther Drug Carrier Syst. 1992; 9: 135-187.
7. Liu D, Mori A, Huang L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta. 1992; 1104: 95-101.
8. Nagayasu A, Shimooka T, Kinouchi Y, Uchiyama K, Takeichi Y. Effects of fluidity and vesicle size on antitumor activity and myelosuppressive activity of liposomes loaded with daunorubicin. Biol Pharm Bull. 1994; 17: 935-939.
9. Jain RK. Barriers to drug-delivery in solid tumors. Sci Am. 1994; 271: 58-65.
10. Choi Y, Baker JR. Targeting cancer cells with DNA-assembled dendrimers: A mix-and-match strategy for cancer. Cell Cycle. 2005; 4: 669-671.
11. Jaracz S, Chen J, Kuznetsova LV. Recent advances in tumortargeting anticancer drug conjugates. Bioorg. Med Chem. 2005; 13: 5043-5054.
12. Tabata Y, Murakami Y, Ikada Y. Tumor accumulation of poly(vinyl alcohol) of different sizes after intravenous injection. J Controlled Release. 1998; 50: 123-133.
13. Zalipsky S, Mullah N, Harding JA. Poly (ethylene glycol)-grafted liposomes with oligopeptide or oligosaccharide ligands appended to the termini of the polymer chains. Bioconjugate Chem. 1997; 8: 111-118.
14. Reddy JA, Allagadda VM, Leamon CP. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr. Pharm. Biotechnol. 2005. 6, 131–150. C. P. Leamon, J. A. Reddy, Folatetargeted chemotherapy. Adv Drug Delivery Rev. 2004; 56: 1127-1141.
15. Roy EJ, Gawlick U, Orr BA. Folate-mediated targeting of T cells to tumors. Adv Drug Delivery Rev. 2004; 56: 1219-1231.
16. Choi Y, Thomas T, Kotlyar A, Islam MT. Synthesis and functional evaluation of DNAassembled polyamidoamine (PAMAM) dendrimer clusters with cancer cellspecific targeting. Chem Biol. 2005; 12: 35-43.
17. Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer- science driving clinical progress. Nat. Rev Cancer 2005; 5: 549-467.
18. Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004; 5: 292-302.
19. Lin MZ, Teitell MA, Schiller GJ. The evolution of antibodies into versatile tumor-targeting agents. Clin Cancer Res. 2005; 11: 129-138.
20. Zhang JY. Tumor-associated antigen arrays to enhance antibody detection for cancer diagnosis. Cancer Detect Prev. 2004; 28: 114-118.
21. Kontermann RE. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol Sin. 2005; 26: 1-9.
22. Trail PA, King DH, Dubowchik GM. Monoclonal antibody drug immunoconjugates for targeted treatment of cancer. Cancer Immunol Immunother. 2003; 52: 328-337.
23. McDonald GC, Glover N. Effective tumor targeting: Strategies for the delivery of armed antibodies. Curr Opin Drug Discov Devel. 2005; 8: 177-183.
24. Govindan SV, Griffiths GL, Hansen HJ, Horak ID, Goldenberg DM. Cancer therapy with radiolabeled and drug/toxinconjugated antibodies. Technol Cancer Res Treat. 2005; 4: 375-392.
25. FitzGerald DJ, Kreitman R, Wilson W, Squires D. Recombinant immunotoxins for treating cancer. Int J Med Microbiol. 2004; 293: 577-582.
26. Sandvig K, Grimmer S, Iversen TG, Rodal K, Torgersen ML. Ricin transport into cells: Studies of endocytosis and intracellular transport. Int J Med Microbiol. 2000; 290: 415-420.
27. Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004; 44: 361-370.
28. Gruaz-Guyon A, Raguin O, Barbet J. Recent advances in pretargeted radioimmunotherapy. Curr Med Chem. 2005; 12: 319-338.
29. Barth RF, Adams DM, Soloway AH. Boronated starburst dendrimermonoclonal antibody immunoconjugates: Evaluation as a potential delivery system for neutron capture therapy. Bioconjugate Chem. 1994; 5: 58-66.
30. Roberts JC, Adams YE, Tomalia DA, Mercer-Smith JA. Using starburst dendrimers as linker molecules to radiolabel antibodies. Bioconjugate Chem. 1990; 1: 305-308.
31. Singh P. Terminal groups in Starburst dendrimers: Activation and reactions with proteins. Bioconjugate Chem. 1998; 9: 54-63.
32. Kobayashi H, Sato N, Saga T, Nakamoto Y, Ishimori T. Monoclonal antibodydendrimer conjugates enable radiolabeling of antibody with markedly high specific activity with minimal loss of immunoreactivity. Eur J Nucl Med Mol Imaging. 2000; 27: 1334-1339.
33. Fischer-Durand N, Salmain M, Rudolf B. Synthesis of metalcarbonyl- dendrimer-antibody immunoconjugates: Towards a new format for carbonyl metallo immunoassay. Chem Bio Chem. 2004; 5: 519- 525.
34. Thomas TP, Patri AK, Myc A, Myaing MT. In vitro targeting of synthesized antibody-conjugated dendrimer nanoparticles. Biomacromolecules. 2004; 5: 2269-2274.
35. Patri AK, Myc A, Beals J, Thomas TP, Bander NH. Synthesis and in vitro testing of J591 antibody-dendrimer conjugates for targeted prostate cancer therapy. Bioconjugate Chem. 2004; 15: 1174-1181.
36. Kolhe P, Khandare J, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials. 2006; 27: 660-669.
37. Nigavekar SS, Sung LY, Llanes M, El-Jawahri A, Lawrence TS. 3H Dendrimer nano nanoparticles organ/tumor distribution. Pharm Res. 2004; 21: 476-483.
38. Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) StarburstTM dendrimers. J Biomed Mater Res. 1996; 30: 53-65.
39. Kobayashi H, Wu C, Kim MK, Paik CH. Evaluation of the in vivo biodistribution of indium-111 and yttrium-88 labeled dendrimer-1B4MDTPA and its conjugation with anti-Tac monoclonal antibody. Bioconjugate Chem. 1999; 10: 103-111.
40. Mamede M, Saga T, Kobayashi H, Ishimori T. Radiolabeling of avidin with very high specific activity for internal radiation therapy of intraperitoneally disseminated tumors. Clin Cancer Res. 2003; 9: 3756-3762.
41. Wiwattanapatapee R, Carren˜o-Go´mez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: A potential oral delivery system? Pharm Res. 2000; 17: 991-998.
42. Wilbur DS, Pathare PM, Hamlin DK, Buhler KR. Biotin reagents for antibody pretargeting. 3. Synthesis, radioiodination, and evaluation of biotinylated starburst dendrimers. Bioconjugate Chem. 1998; 9: 813-825.
43. Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Controlled Release. 2000; 65: 133-148.
44. Yang W, Barth RF, Wu G, Bandyopadhyaya AK, Thirumamagal BTS. Boronated epidermal growth factor as a delivery agent for neutron capture therapy of EGF receptor positive gliomas. Appl Radiat Isot. 2004; 61: 981-985.
45. Kobayashi H, Brechbiel MW. Dendrimer-based macromolecular MRI contrast agents: Characteristics and application. Mol Imaging. 2003; 2: 1-10.
46. Stiriba SE, Frey H, Haag R. Dendritic polymers in biomedical applications: From potential to clinical use in diagnostics and therapy. Angew Chem Int Ed. 2002; 41: 1329-1334.
47. Kaminaga T, Takeshita T, Kimura I. Role of magnetic resonance imaging for evaluation of tumors in the cardiac region. Eur Radiol. 2003; 13(4): 1-10.
48. Graaf PD, Barkhof F, Moll AC, Imhof SM. Retinoblastoma: MR imaging parameters in detection of tumor extent. Radiology. 2005; 235: 197-207.
49. Ogan MD, Schmiedl U, Mosely ME, Grodd W. Albumin labeled with Gd-DTPA. An intravascular contrastenhancing agent for magnetic resonance blood pool imaging:Preparation and characterization. Invest Radiol. 1987; 22: 665-671.
50. Margerum LD, Campion BK, Koo M, Shargill N, Lai J. Gadolinium(III) DO31 macrocycles and polyethylene glycol coupled to dendrimers. Effect of molecular wieght on physical and biological properties of macromolecular magnetic resonance imaging agents. J Alloys Compd. 1997; 249: 185-190.
51. Konda SD, Wang S, Brechbiel M, Wiener EC. Biodistribution of a 153 Gd-folate dendrimer, generation ¼ 4, in mice with folate-receptor positive and negative ovarian tumor xenografts. Invest Radiol. 2002; 37: 199-204.
52. Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents. Magn Reson Med. 2001; 46: 781-788.
53. Kobayashi H, Kawamoto S, Jo SK. Macromolecular MRI contrast agents with small dendrimers: Pharmacokinetic differences between sizes and cores. Bioconjugate Chem. 2003; 14: 388-394.
54. Guccione S, Li KC. Molecular imaging and therapy directed at the neovasculature in pathologies. How imaging can be incorporated into vascular-targeted delivery systems to generate active therapeutic agents. IEEE Eng Med Biol Mag. 2004; 23: 50-60.
55. Onitsuka K, Fujimoto M, Kitajima H. Convergent synthesis of platinum-acetylide dendrimers. Chem Eur J. 2004; 10: 6433-6446.
56. Barth RF, Soloway AH. Boron neutron capture therapy of primary and metastatic brain tumors. Mol Chem Neuropathol. 1994; 21: 139-154.
57. Liu L, Barth RF, Adams DM, Soloway AH. Bispecific antibodies as targeting agents for boron neutron capture therapy of brain tumors. J Hematother. 1995; 4: 477-483.
58. Alam F, Soloway AH, Barth RF, Mafune N, Adams DM. Boron neutron capture therapy: Linkage of a boronated macromolecule to monoclonal antibodies directed against tumorassociated antigens. J Med. Chem 1989; 32: 2326-2330.
59. Barth RF, Wu G, Yang W, Binns PJ, Riley KJ, Patel H. Neutron capture therapy of epidermal growth factor (þ) gliomas using boronated cetuximab (IMC-C225) as a delivery agent. Appl Radiat Isot. 2004; 61: 899-903.
60. Fu, HL; Cheng SX, Zhang XZ (2007). "Dendrimer/DNA complexes encapsulated in a water soluble polymer and supported on fast degrading star poly(DL-lactide) for localized gene delivery". Journal of Control Release(Key Laboratory of Biomedical Polymers of Ministry of Education, Department of Chemistry, Wuhan University) 124 (3): 181–188..
61. Dendrimer Design Using Cu(I)-Catalyzed Alkyne-Azide Click Chemistry, G. Franc, A. Kakkar Chem. Comm., 2008, pp 5267 - 5276
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









