About Authors:
Masani Jigneshkumar Naranbhai
School Of Science
Biomedical Technology
Jiwaji University, Gwalior
jigneshmasani11@gmail.com
ABSTRACT
A supernumerary marker chromosome is an extra structurally abnormal chromosome of unknown origin. Various factors contribute to the difficulty of clarifying the phenotypicrisks of super numerary marker chromosomes (SMC), including differences inthe size, structure, and origin of marker chromosomes.
All marker chromosomes do not contribute the same risk of recurrence. Therefore characterization of the marker chromosomes is of utmost importance for Genetic Counseling. Different molecular techniques can be used to identify the chromosome material present in the markers. In the present retrospective data analysis of 15,000 karyotypes of all patients referred to the Center of Medical Genetics, Sir Ganga Ram Hospital from 1998 to 2012. For constitution chromosome abnormality detection, we found fifteen (0.01%) patients had presence of the SMC. Of these fifteen, eight markers had been characterized after FISH was introduced in the year 2004. This contributed to personalized Genetic Counseling and help for future pregnancies.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1411
INTRODUCTION
WHAT IS CYTOGENETICS
Cytogenetics is the study of chromosomes structure, function, behavior and pathology. The study of human chromosome plays a role in the diagnosis, prognosis, and monitoring of treatment involving conditions seen not only by medical geneticist and genetics counselors but also by pediatricians obstetrician/gynecologist oncologist.
AIMS AND GOALS OF CYTOGENETICS STUDIES
1. Etiological studies
2. Assortment of morbidity and mortality in the affected
3. Genotype phenotype correlation
4. Recurrent risk and reproductive fitness studies
5. Break point studies in structural rearrangement
HISTORY
· The Cytogenetic era started when the first chromosomes were visualized through microscope 1880.
· The first evaluable human metaphase chromosomes spread for diagnostics was published almost six decades ago (Tijo and Levan 1956).
· This followed the detection of the first chromosomes abnormalities such as Down syndrome (Lejeune 1959), and the detection of the presence of sSMC (Ilberry et al. 1961).
· Nowadays, the G bands by trypsin- Giemsa stain (GTG) banding technique (Seabright 1971) is still the starting point and gold standard of all cytogenetic techniques. It is relatively cheap, easy to perform, and gives an overview of the whole human genome. However marker chromosomes are not always easy to identify even with G-banding. Therefore Molecular cytogenetic techniques have to be used to characterize a marker chromosome.
COMMON INDICATION FOR CYTOGENETIC ANALYSIS
PRECONCEPTION
1. Unexplained infertility or sterility
2. Exposure to potential teratogenic agents
3. Two or more pregnancy loss
4. A previous child with a genetic disorder or birth defect (e.g. down syndrome)
NEONATES AND CHILDREN
1. A history of IUGR or failure to throne
2. Abnormal growth patterns
3. Ambiguous or abnormal genitalia early onset of puberty
4. Microcephaly or Carniotenosis
5. Hypotonia, hypertonic
6. Dysmorphic feature
7. Abnormal body and limb proportions
8. Major or minor congenital abnormalities
9. Unusual behavior
ADOLECENT AND ADULTS
1. Abnormal sexual maturation
2. Amenorrhea delayed puberty
3. Growth retardation
4. Excessive tall stature
5. Mental retardation
6. Hematological disorder instability syndrome
PRENATAL
1. A women who will be 35 years of age or older at delivery.
2. A women or her partner who carries a chromosomal rearrangement abnormality or a single gene disorder.
3. Couples with a family history of neural tube defects.
4. Women with an abnormal level of maternal serum.
5. Women who exposed to infectious disease radiation drug or other environmental agents during pregnancy.
6. Family history of a chromosomal syndrome maternal anxiety.
CHROMOSOMES
Chromosomes are thread-like structures located inside the nucleus. Each chromosome is made of protein and a single molecule of deoxyribonucleic acid (DNA). Passed from parents to offspring, DNA contains the specific instructions that make each type of living creature unique.
The term chromosome comes from the Greek words for color (chroma) and body (soma). Scientists gave this name to chromosomes because they are cell structures, or bodies, that are strongly stained by some colorful dyes used in research.
(genome.gov)
Chromosomes were first observed in plant cells by Karl Wilhelm von Nageli in 1842. Their behavior in animal (salamander) cells was described by Walther Flemming, the discoverer of mitosis, in 1882. The name was coined by another German anatomist, von Waldeyer in 1888.
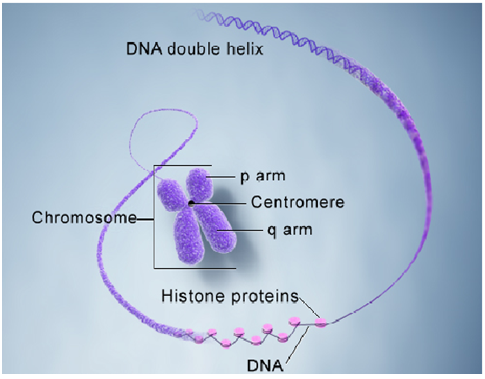
SHAPE
Theyare basically rod shaped in outline.
The smaller larger chromosomes may appear dots with rounded or oval outline.
The larger chromosomes may be straight or bent to become arc like.
SIZE
Thesize of the normal chromosomes varies from 0.5 – 30 um in length and 0.2 – 0.3 um in breadth.
STRUCTURE
Basicallythe chromosomesconsist of centromere, secondary constrictions nucleolus organizer, telomere and satellites.
CHROMOSOME NUMBERS
· All animals have a characteristic number of chromosomes in their body cells called the diploid (or 2n) number.
· These occur as homologous pairs, one member of each pair having been acquired from the gamete of one of the two parents of the individual whose cells are being examined.
· The gametes contain the haploid number (n) of chromosomes.
|
Diploid numbers of some commonly studied organisms |
|
|
Homo sapiens(human) |
46 |
|
Mus musculus(house mouse) |
40 |
|
Saccharomyces cerevisiae (budding yeast) |
32 |
|
Zea mays (corn or maize) |
20 |
|
Drosophila melanogaster (fruit fly) |
8 |
CENTROMERE
Thechromosomes have constriction during division the centromere is functional,therefore the centromere may the locus of genes for mitotic and meiosis activity.
Dependingupon the position ofcentromere chromosomes are dividedinto-
Metacentric-centromere in the middle chromosomes appear V shaped
Submetacentic-centromere is situated some distance away from the middle.
Telomeres-The tip of the chromosomes is called telomere. It has a unique property in that it prevents the ends of chromosome from sticking together.
Telomeres are the special modified regions of chromosomes for attachment to nuclear envelope.
Inhuman Chromosomesare the structures in each of the body’s cells that carry the geneticinformation. Theycome in pairs, one from each parent, and are numbered 1 to 22 approximately from largest to smallest. The sex chromosomes are the last pair, called X & Y in males and X & X in females. Each chromosome has a short (p) arm and a long (q) arm. So we humansusually have 46 chromosomes.
Geneticistswrite this in a code (karyotype)format: 46, XX (for a female)
Or 46, XY (for a male)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
KARYOTYPE
It is the chromosome complement of a cell, individual or group of similar individuals that provides description of number, types and other characteristic of chromosome.
Karyotype is described in the form of ideogram.
Ideogram is a photograph or diagram of metaphases chromosomes of an organism arranged in homologous pair according to their length, thickness and position of centromere, length of arm, shape and other characteristics.
The sex chromosomes are usually placed at the end. Cultured cells growing under aseptic condition are generally used for karyotyping.
Importance
· Any changes in the chromosome number (extra or deficient) are detected immediately.
· Chromosomal aberrations or abnormalities are found out.
· Comparison of karyotype can indicate relationship amongst species.
HUMAN KARYOTYPE
The complete set of chromosomes in the cells of an organism is its karyotype. It is most often studied when the cell is at metaphase of mitosis and all the chromosomes are present as dyads.
The karyotype of the human female contains 23 pairs of homologous chromosomes:
· 22 pairs of autosomes
· 1 pair of X chromosome
The karyotype of the human male contains:
· the same 22 pairs of autosomes
· one X chromosome
· one Y chromosome
Human autosomes are numbered and distinguishaed morphologically on the basis of their relative size, population of centromere.
According to ‘Denver system’ of classification the 22 pairs of human chromosomes are placed in seven groups, A to G.
Group A: It consists of three large metacentric chromosomes numbered 1-3.
Group B: The group includes two pairs of submetacentric chromosomes numbered 4 and 5. They are larger but shorter than those of group A.
Group C: It has medium sized submetacentric chromosomes no. 6 to 12.
Group D: The group includes medium sized acrocentric set chromosomes 13 to15.
Group E: The group has short submetacentic chromosomes 16 to 18.
Group F: It contains short sized metacentric chromosomes 19 and 10.
Group G: The group includes short acrocenrtic set chromosomes with or without satellites numbered 21 and 22.
The X and Y sex chromosomes are commonly placed at the end of ideogram. X chromosome is similar in appearance to C- group of autosomes. Y chromosome has variable length and similar to G- group of autosomes, arise without satellites.
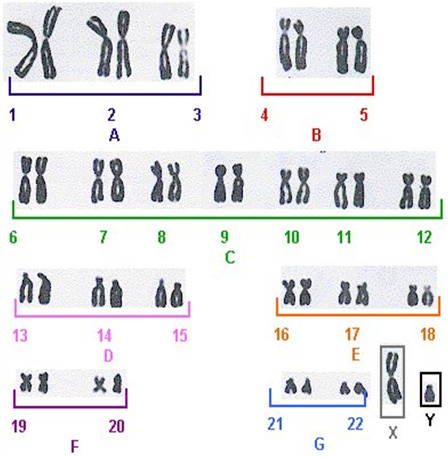
Karyotype of metaphase stage in a normal male.
The procedure of the techniques is given in forthcoming pages.
REVIEW OF LITERATURE
MARKERCHROMOSOME
Marker chromosomeisdefined as anadditionalstructurally abnormal chromosome of unknown origin frequently found in karyotype of cancer patients & patients with constitutional genetics disorders’ [ISCN (International System for human Cytogenetic Nomenclature) (Crolla et al. 1997).It 2009]. It is derived from any of the 24 different chromosomes 1 to 22, an X or a Y chromosome.Single or multiple marker chromosomes often occur in a mosaic form, especially in acquired abnormalities such as Leukemia.
Synonyms for marker chromosomes
Extra Structurally Abnormal Chromosome (ESAC),
Small Supernumerary Marker Chromosome (sSMC),
Small Accessory Chromosome (SAC),
Biosatellite Marker Chromosome,
Supernumerary Ring Chromosome (SRC),
Extra or Additional Marker Chromosome,
Cancer associated Neochromosome, and
Neocentric Marker Chromosome (NMC)
ESACarefound inapproximately1in2,500 newborns(Friedrichand Nielsen, 1974; Bucktonet al., 1985; Warburton,1991).
SMCsare estimated to occur in 0.04%~0.05% of live births.
In the world of 700 crore populations, 1.20 crore individuals carry a marker chromosome: 84 lac (70 %) have normal growth, develop and have no health problem and 36 lac (30 %) have problem with Growth & Development.
Fertility Percentage: ESAC is found in 0.044 % (approx. 27 lac) of the general population.
Prenatal cases: ESAC is found in 0.125 % (approx. 90 lac) during prenatal testing by conventional cytogenetics.
Sex related occurrence: It is 7.5 times higher in male than female.
SHAPES
ESAC can have three different shapes:
(1) Inverted duplicated sSMC
(2) ring-shaped sSMC
(3) Centric minutes-shapes sSMC and
(4) Neocentric sSMC.
Written as karyotype formulas are as ‘inv dup’, ‘r’, ‘min’ and ‘neo’.
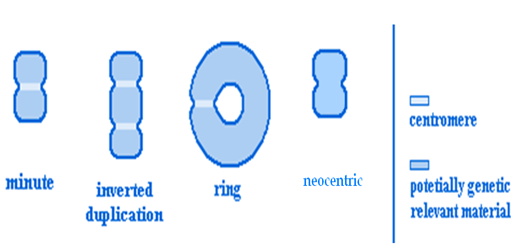
It is more commoninpatientswith congenital abnormalities,abnormal sexual development,or mentalimpairment(Bucktonet al.,1985). The significance of a marker is very variable as it depends on what material is contained within the marker. Fluorescenceinsituhybridization(FISH)hasprovedtobe helpfulinfurtheridentifyingthechromosomaloriginof marker chromosomes(Callen etal.,1992; Danieletal.,1994). However, it is still very difficultto compareclinicalfindingsin patientswith markersofthesame chromosomalorigin.
CORRELATING SYNDROME
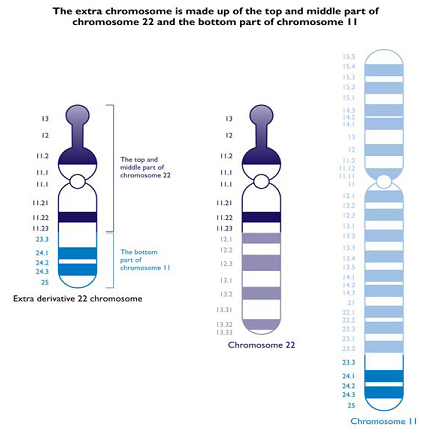
A)Emanuel Syndrome
· Emanuel Syndrome is caused by the presence of an extra chromosome, made up of parts of chromosomes 11 & 22.
· It is known as ‘derivative chromosome 22 syndrome’.
· It can occur in offspring of carriers of the constitutional chromosomal translocation t(11;22)(q23;q11).
· It is caused by the presence of an extra derivative chromosome, which is made up of the top part of chromosome 22 and the bottom part of chromosome 11. Normal individuals have 46 chromosomes, but individuals with Emanuel Syndrome inherit this extra 11/22 chromosome, which gives them 47 chromosomes in total.
Symptoms
· Small head- microcephaly
· Small lower jaw- micrognathia,
· Distinctive facial features
· Cleft palate.
· Males have genital abnormalities.
· Additional heart defects, small kidneys.
· It does not have a cure, but individual symptoms may be treated.
· Assessments of individual systems, such as the cardiovascular, gastrointestinal, orthopedic, and neurological may be necessary to determine the extent of impairment and options for treatment.
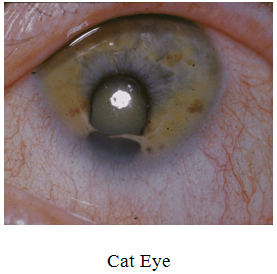
B) Cat Eye Syndrome
· It is the more common name for a condition involving a partial trisomy or tetrasomy of part of chromosome 22.
· A small extra chromosome (humans normally have only 2) made up of the top half of chromosome 22 – the “p” arm, as well as the portion of the long arm of chromosome 22 down to the breakpoint q11.2, is found to be present either three times (trisomy) or four times (tetrasomy ).
· It affects both males and females, and is estimated to occur in 1 in 74,000 individuals. Many individuals born with the syndrome are the only ones in their families who have it.
Symptoms
· Anal atresia (abnormal obstruction)
· Preauricular pits/tags
· Cardiac defects
· Kidney problems
· Short stature
· Scoliosis/Skeletal problems
· Mental retardation
· Micrognathia-smaller jaw
· Cleft palate
Treatment of cat eye syndrome focuses on the symptoms the individual has. Some children may need surgery to repair birth defects in the anus or heart.
Life expectancy is not significantly reduced in those individuals who do not have life-threatening physical problems (such as a severe heart defect).
C) Pallister-KillianSyndrome (PKS)
· Itis also calledMosaic tetrasomy 12p.
· Itis a rare chromosome disorder.
· Itis caused by having a small extra chromosome made up of two copies of the short (p) arm of chromosome 12 in some cells of the body. In these cells there are four copies of this arm of chromosome 12 instead of the usual number of two.
· Theeffects of mosaic tetrasomy 12p are caused by the genes carried on the extra chromosome. However, among people with mosaic tetrasomy 12p the severity of the effects varies enormously. However, people with mosaic tetrasomy 12p often appear to have normal chromosomes in their blood cells, so the diagnosis is usually made from skin cells or cells taken from inside the cheek.
Symptoms
· Floppiness(hypotonia) inbabies
· Developmental delay
· Learningdifficulties
· Delayin developingspeech or absence of speech inbabies and young children
· A distinctive facial appearance includinga high roundedforehead, broadnasal bridge, widely spaced eyes and a large mouthwith a thin upper lip and a ‘coarse’ look to the face,sparsehair or bald patches around the templesandthin or sparse eyebrows.
D) Isochromosome 18p Syndrome
· Tetrasomy 18p is a very rare chromosomal disorder and is the result of a spontaneous mutation early in embryonic development in most of the cases.
· This condition is characterized by the presence of a supernumerary 18p isochromosome (i(18p)) in all or some cells of the affected individual.
· It has a prevalence of 1/180000 live births and affects both genders equally.
Symptoms
· Mental retardation
· Microcephaly
· Craniofacial anomalies
· skeletal findings
· Hypertonia
· Scoliosis or kyphosis
· occasionally renal and cardiac malformations.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
E) Supernumeraryring chromosome
· Asupernumerary ring chromosome(SRC)means that there is a tiny extra part of a chromosomein all or some of the cells of the body. In addition to the 46 chromosomes thateveryone has, people with supernumerary ring chromosomehavea small extra chromosomemade from any of the 24 chromosomes.
· Theextra chromosome usually consists of a small piece that has broken off near the middle of the chromosome and the ‘sticky’ ends have curledup to join and form a ring.
· No-oneknows yet just how ringsform, but the picture on the bottom shows one possibility. Mostring chromosomes occurwhen both parents have normal chromosomes.
· Geneticistscallthis de novo (dn).
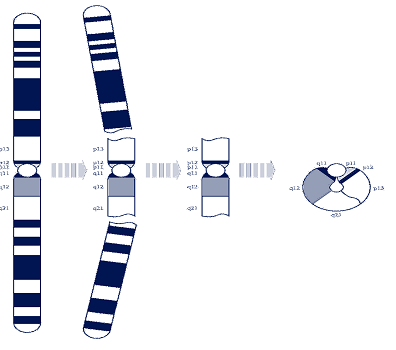
· These rings are caused by a change that occurredwhenthe parents’ sperm or egg cells were formed. Chromosomesmust breakand rejoin when egg and sperm cells are formed butthis only occasionally leads toproblems.
· Thebreaking and rejoining that causedthe ring to form during egg orspermproduction is part of a natural process and as a parent you cannot change or control it.
· Somesupernumerary ring chromosomes are inherited. The mother or the father has the same ring chromosome. Ablood test to check the parents’ chromosomeswill show if the ring is inheritedor not.Childrenfrom all parts of the world and from all types of background have supernumeraryrings. No environmental, dietary or lifestylefactors are known to cause them.There is nothing that either parent did beforeor during pregnancy that can beshownto have caused the ring to occur or equally nothing could have been done to preventit.
· Mostpeople with a ring chromosome have mosaicism.Onecell line has the normal make-upof 46 chromosomes and thesecond cell line has the extra ring chromosome.When cells divideduring development, there is a tendency for the cells with the ring chromosome tobe dropped and this leads to mosaicism.
FISH TECHNIQUE
Fluorescence In Situ Hybridisation (FISH) technique can be used for detection of Marker Chromosome. FISH is a cytogenetic technique developed by biomedical researchers in the early 1980s. FISH is a targeted approach where fluorescent probes bind to only those parts of the chromosome with which they show a high degree of sequence complementarity. Fluorescence microscopy can be used to find out where the fluorescent probe bound to the chromosomes. FISH uses small DNA strands called probes that have a fluorescent label attached to them. The probes are complementary to specific parts of a chromosome. When DNA is heated, the patient’s two DNA strands break apart, or denature, and the probes are able to hybridise to their complementary sequence in the patient’s DNA (see diagram below). If a small deletion is present in the region complementary to the probe, the probe will not hybridise. If duplication is present, more of the probe is able to hybridise.
The advantages of FISH that it is able to detect many small deletions, duplications and rearrangements that are not visible with standard microscope analysis and a diagnosis from FISH may avoid your child having to undergo many other tests.
The benefits of FISH it may help you and your doctor watch for common health problems associated with your child’s chromosome imbalance, It may help to predict what to expect as your child gets older, It may show which specific genes are included in your child’s deletion or duplication. If the gene(s) has been associated with a particular feature or health problem it may help to guide management or treatment for your child, it can help you to obtain specialist services for your child.
You can choose to join a support group to meet other parents facing similar challenges, Parents and other family members can be tested to see if they are carriers of changes in their DNA that put them at risk of having more children with a chromosome change.
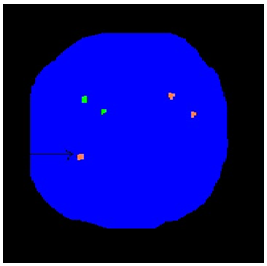
[Fig. FISH technique to show extra Y chromosome]
Objective of Study:
Thereare three different situationsin which an sSMC may be diagnosed
· Inpregnancy, during prenatal screening and diagnosis.
· Ina newborn baby due to clinical problems and/or unusual facial features. (knownas dysmorphic)
· Inan otherwise healthy adult who has experienced problems with fertility.
To conduct a retrospective analysis of fifteencaseswith an extra structurally abnormal chromosomes in cultured lymphocytes from 15000 bloodsamples referred for karyotyping at Center of medical genetics, Sir Ganaga Ram hospital, new Delhi over the last ten years. Thiswill establish the frequency of the marker chromosomes in the India.
|
Karyotyping |
Chromosome origin |
Diagnosis |
|
46,XY[33]/46,X,+ESAC[7] |
Y chromosome |
Blood, Verify to Marker Chromosome |
|
47,XY,+ESAC
|
chromosome 18 |
Blood, Developmental Delay |
|
47,XY,+ESAC
|
chromosome 18 |
Blood, R/o Down Syndrome |
|
47,XX,+ESAC |
Marker not characterized |
Blood, Developmental Delay + Dysmorphism |
Frequency of the Marker Chromosomes in Sir Ganga Ram Hospital, New Delhi.
|
Sr. No. |
Sample |
Marker Chromosomes find |
Frequency in % |
|
1. |
Blood |
18 |
0.050 |
|
2. |
Amniotic Fluid |
5 |
0.014 |
In this hospital, 35,700 total patients examined for Cytogenetics Laboratory. Very rare patients are to characterize as the marker chromosome. In Blood samples 18 cases and Amniotic fluid samples 5 cases are characterized.
MATERIALS AND METHODS
CYTOGENETICS PROCEDURE
The study of chromosomes using traditional cytogenetics techniques requires cells that are actively dividing. Chromosomes are individually distinguishable under the light microscopy only during cell division and best examined during metaphase. Metaphase chromosomes can be obtained from specimens that contain spontaneously dividing all or ones that are cultured and chemically induced to divide in vitro.
Constitutional chromosomal patterns are best study by peripheral blood studies. Hence, this is the best commonly used tissue for cytogenetics investigation.
The constituents of peripheral blood are RBC (erythrocytes), WBC (leucocytes) and platelets (thrombocytes).
The WBC includes –
· Neutrophils
· Eosinophils
· Basophils
· Lymphocytes
· Monocytes
For Routine cytogenetics analysis, lymphocytes are stimulated.
In the present study 15 cases which were referred to the cytogenetics laboratory, Center of medical Genetics at Sir Ganga Ram Hospital was subjected for karyotyping.
Chromosomepreparationandbanding
Metaphase chromosomeswere preparedin the standardway fromhumanperipheralblood.R-,C-,andG-bandingwereperformedaccordingto thetechniques ofDutrillaux and Lejeune(1971), Sumner(1972), and Sea- bright (1973).Twenty metaphasespreadswereanalyzedinthepatient andtheirparents.
KARYOTYPING PROTOCOL
Equipment and reagents
Horizontal Laminar Flow
15ml sterile disposable PS Centrifuge tubes
Test-tube rack
10ml sterile syringes
2ml sterile syringes
Incubator at 37 degrees C
Standard Centrifuge
Standard Slides
Deep-freezer –20 degrees C
Refrigerator
Fume hood
Pasteur pipettes
Vortex mixer
Growth Media
Large number of media is available for tissue culture and their use depends on tissue to be grown. The basal medium is supplemented with serum for growth factors and proteins.
Usually fetal bovine serum ornew born calf serum is used. Other supplements for growth are antibiotics which are added to the culture media to retard the growth of microorganism.
Mitogenic Stimulants (Mitogens)
Some cells, particularly mature lymphocytes, do not spontaneously undergo cell division and must be stimulated to divide by the addition of an appropriate mitogen to cell culture.
Phytohaemagglutinin (PHA) is an extract of red kidney beans that stimulates division on primarily of T-lymphocytes. Cell division starts 18 hrs. For routine peripheral blood cultures, 72 hrs is usually optimal.
PB Max karyotyping medium (Growth Media and Mitogens)
This complete media consists of RPMI-1640, Fetal bovine serum, L-glutamine, Gentamycin sulfate and Phytohaemagglutinin.
Colchicine
It is used to obtain adequate number of cell in metaphase. It basically arrests the cell growth in metaphase (Mitotic Arrest).
It binds to the protein tubulin, obstructing the formation of spindle fibers or destroying those already present. This prevents separation sister chromatids in anaphase, thus collecting cells in metaphase.
Colchicine 5mg
Distilled water 5ml
Ethidium Bromide
Ethidium Bromide 5mg
Distilled water 10ml
Hypotonic Solution (0.075M KCl)
The hypotonic solution has a lower salt concentration than the cell cytoplasm, allowing water to move into the cell by osmosis. This swells the cell and is critical for adequate spreading of the chromosome on the slide.
Potassium Chloride 0.56g
Distilled water 100ml
Fixative
The commonly used fixative is a freshly prepared and chilled mixture of Methanol and Glacial Acetic Acid in 3:1 proportion. In the mixture of methanol fix the cell and Glacial Acetic Acid is lyses the RBC cells.
Methanol 30ml
Acetic acid 10ml
STEPS
1) SPECIMEN COLLECTION
The blood used for cytogenetic analysis is obtained by venipucture.
The venipucture is performed on the antecubital vein.
In neonates or young children, blood can be collected by scalp vein or femoral puncture.
Peripheral blood samples should be collected in sterile syringes or vacuum tubes containing preservative-free sodium heparin.
How to collect
The skin over the vein is cleaned with 70% alcohol and blood is collected in heparinised syringe; alternatively it can be collected in a plain, sterile syringe and transferred to a vial with 0.5 ml of heparin provided by the laboratory.
Precautions
a) Before the vein is located, ensure that a labeled syringe with needles and labels are ready.
b) Always recommended to use sterile disposable needle and syringe or vacutainer.
c) Always wear gloves while handling any samples. Treat all samples as infectious material. A spill of any sample must be treated with sodium hypochlorite.
d) Colchicine is toxic by skin contact, ingestion and inhalation and may cause irreversible effects.
e) The blood used for cytogenetics analysis should not clot.
Added information:
Blood from infants less than 1 year old contains more protein than that from adults and older children, and therefore, less whole blood should be used to get good quality, clean preparations.
Suboptimal samples
Occasionally, samples arrive in the wrong tube, most often an EDTA tube which is required for molecular genetic test. The anti-coagulation properties of EDTA are derived from its ability to chelate Ca+2. Calcium ions are required for both blood clotting and PHA stimulation of lymphocytes. Thus, while coagulation is prevented, the mitotic index obtained from such samples is considerably reduced. Samples can be partially 'rescued' by washing out the EDTA; heparin is incorporated into the medium, in order to prevent clotting once the EDTA is removed. Washed EDTA blood appears to contain fewer lymphocytes, thus addition of 1ml of whole blood should be used to inoculate cultures of 5ml.
2) CULTURE INITIATION OF AND PLANTING
a) Log in the name, laboratory no., and diagnosis of the patient in the Planting register.
b) Aliquot 5 ml of Karyomax into prelabelled 15ml centrifuge tubes.
c) Using a 2ml syringe, inoculates the tube with an appropriate amount of whole Blood, and mix well (adult blood, 0.5ml; fetal or neonatal blood, 0.3ml.)
d) Incubate the culture at 37°C for 72 hrs.
e) Add 1drop of Colchicine (5mg in 5ml) and 2 drops of Ethidium Bromide. (5mg in 10ml)
f) Incubate at 37°C for a further 23 min.
g) Harvest the sample.
3) HARVESTING
a) Centrifuge the tubes. Discard the supernatant and add 5ml of hypotonic Solution (0.56%KCl).
b) Incubate the tubes at 37degrees C for 17minutes.
c) Centrifuge the cells. Discard supernatant.
d) Add 5ml fixative, initial 1 ml drop by drop.
e) Repeat steps 3 and 4
f) Keep the cells in fixative in the freezer, overnight.
4) SLIDES PREPARATION
Slides make is the best done after the cells have been in fixatives at 4oC overnight. However, in case of urgency satisfactory results can be achieved from freshly harvested material.
For making slides,
a) Remove culture tubes from the refrigerator.
b) Spin the tubes.
c) Wash the cells with 5ml fixative again.
d) Discard the supernatant.
e) Gently resuspend the cells in 0.05ml fixative.
f) Drop 1 or 2 drops of cell suspension on the prechilled slides.
g) Dry the slide on the hotplate 56 to 60oC.
h) Mature the prepared slides at 37oC overnight.
5) CHROMOSOME BANDING AND STAINING
Prior to 1970’s human chromosomes were ‘solid’ stained using orcein or other stains with an affinity for chromatin but they provide with little information. Simple aneuploids could be recognized but structural aberrations were difficult to recognize.
A large number of banding and staining techniques have since been developed. These can be divided into two broad categories-
a) Those that produce specific alternating bands along the length of each entire chromosomes.
b) Those that stain a specific region of some or all chromosomes
c) But in laboratories, we use mainly G-Banding.
G-Banding (Giemsa Banding)
G-Banding is the most widely used banding method. GTG banding (G band produced with Trypsin and Giemsa) is one of the several G-band techniques.
With this method, prepared fixed cells slides are treated with enzyme Trypsin and then stained with Giemsa. This produces a series of light and dark bands that allow for the positive identification of each chromosome. The dark bands are A – T rich, late replicating, heterochromatic regions of the chromosome, whereas the light bands are C – G rich, early replicating, euchromatic region.
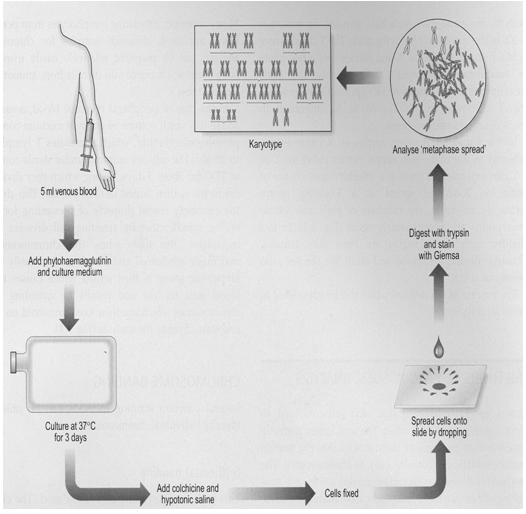
Procedure of Blood Karyotyping
6) OBSERVATION OF SLIDES, KARYOTYPING AND INTERPRETATION OF THE RESULTS
Under low power magnification (10X), select the appropriate metaphase by microscope. And then under high power magnification (100X), the number of chromosome counted.
Count chromosomes in at least 10 to 20 metaphases Karyotype at least two to six metaphases using the computerized image analysis Software for chromosome analysis: Cytovision by DSS imaging/Olympus
A normal karyotype consists of 46 chromosomes. Chromosome Banding is used to identify both normal and rearranged chromosomes and to define chromosome breakpoints, using an International System for Human Cytogenetic Nomenclature (2009), referred to as “ISCN 2009 “. It is the report of the standing committee on human cytogenetic nomenclature edited by D.G. Harnden and H. P. Klinger. High resolution banded chromosomes are classified at least at 550 band level.
· RoutineLymphocyte cultures were performed followedby GTG Banding and karyotyping. Chromosome analysis was done and reports were written using the ISCN 2009
· Fluorescence In situ hybridization was done whenever possible to characterize the marker chromosomes.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS
PRESENT STUDTY
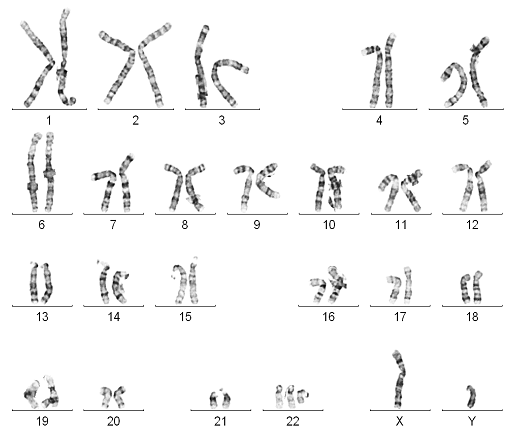
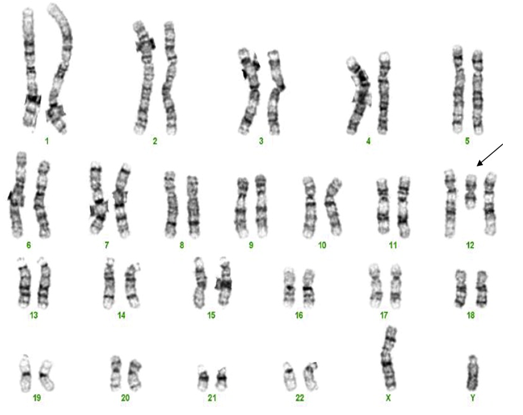
1. Karyotyping of one normal female: 46,XX (image attached)
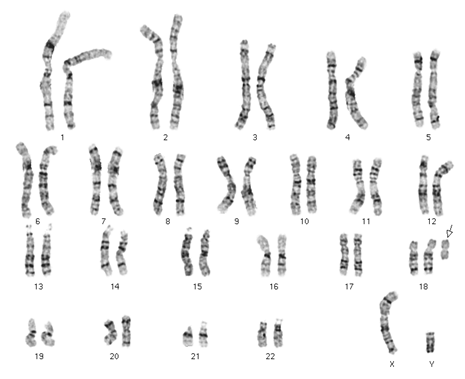
2. Karyotyping of one normal male: 46,XY (image attached)
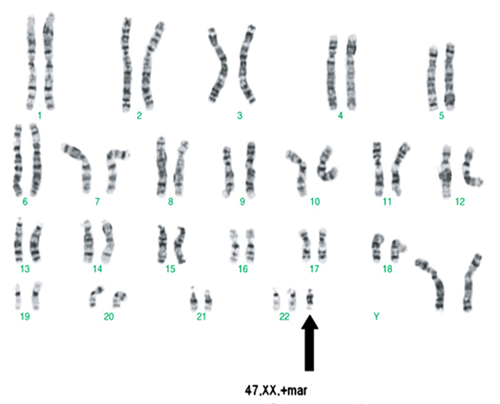
3. Karyotyping of one case of marker chromosome female: 47,XX,+mar (image attached)
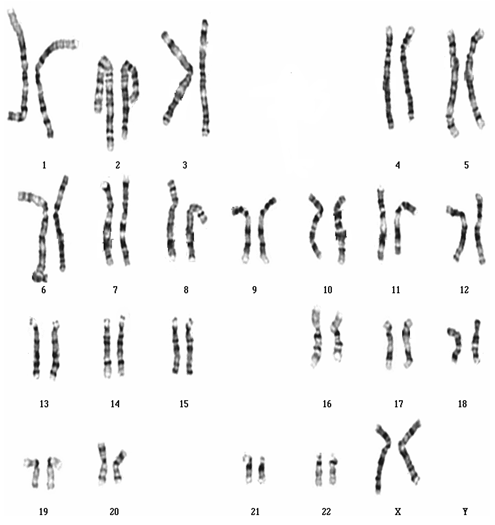

Karyotyping of one normal female: 46,XX
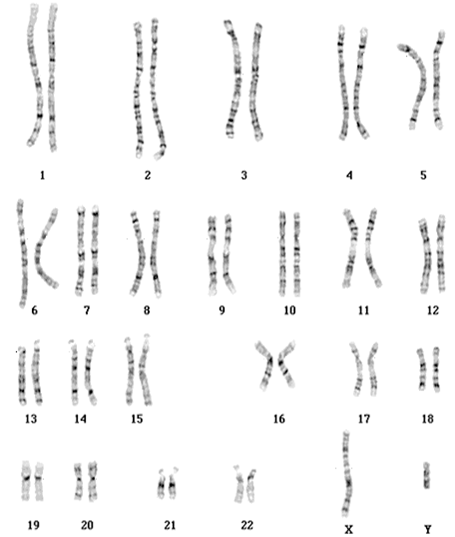
Karyotyping of one normal male: 46,XY
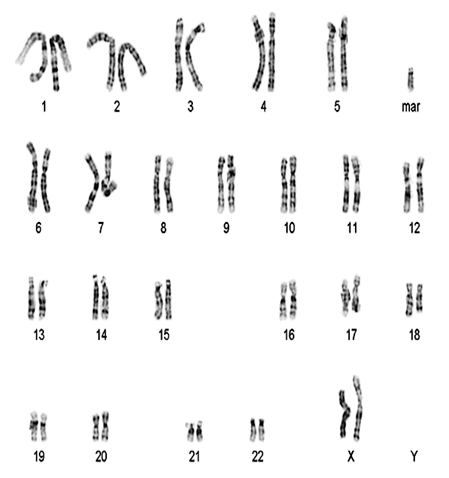
Karyotyping of marker chromosome female: 47,XX,+mar
|
|
|
TABLE RESULT |
|
|
|
Case No. |
Age/Sex |
Clinical details |
Karyotype |
Characterized |
|
1 |
2.5y/M |
Dysmorphic |
47,XY,+ESAC |
invdup22 (pterq11) |
|
2 |
02y/M |
Dysmorphic |
47,XY,+ESAC |
i18p |
|
3 |
01y/M |
Developmental delay |
47,XY,+ESAC |
i18p |
|
4 |
02y/F |
Developmental delay |
47,XX,+ESAC |
i18p |
|
5 |
35y/M |
Partner has recurrent abortions |
46,XY[33]/ 46,X,+ESAC[7] |
iYq11 |
|
6 |
24y/F |
Habitual abortions |
47,XX,+ESAC |
inv15q11 |
|
7 |
21y/M |
Partner has missed abortions |
47,XY,+ESAC |
invdup15q11 |
|
8 |
02y/M |
Developmental delay |
47,XY,+ESAC[2]/ 46,XY[38] |
low level mosaicism not characterized |
|
9 |
9 mths/F |
Developmental delay |
47,XX,+ESAC |
not characterized |
|
10 |
6.5y/F |
Dysmorphic |
(146,XX[18]/ 47,XX,+mar[2] |
low level mosaicism not characterized |
|
11 |
8.5y/M |
Autism |
46,XY[3]/ 47,XY,+ESAC[7] |
small ring not characterized |
|
12 |
35y/F |
2 missed abortion |
47,XX,+ESAC |
minute ring, not characterized |
|
13 |
23y/F |
Hypotonia / Prader willi |
47,XX,+ESAC |
minute ring |
|
not characterized |
||||
|
14 |
30y/M |
Partner has H/o missed |
47,XY,+ESAC |
not characterized |
|
15 |
01y/M |
Developmental delay |
46,XY[48]/ 47,XY,+ESAC[2] |
low level mosaic not characterized |
Out of 15,000 referrals for constitutional chromosomal abnormalities, the marker chromosome was found in 15 samples (Table No.1) Out of fifteen marker chromosomes, three were i(18)p11, two were i(15)p11, one was i(22)p11, one characterized as i(Y)p11, three were small rings, and three had low level mosaicism and two metacentric markers. The last eight were very old cases karyotyped before the introduction of FISH and therefore could not be characterized.
DISCUSSION
To gain hands on experience in the cytogenetic techniques of planting of cultures, harvesting them, and chromosomes analysis, in the present study.
(1) Lymphocytes from whole blood of one normal female and one normal male were cultured and harvested.
(2) Karyotype and image analysis was done in one normal female, one normal male and one abnormal case: a case of marker chromosome.
The birth prevalence of SMC is estimated at 0.14 to 0.72per 1000 (Gardner and Sutherland).
The frequency of SMC estimated in the present study was 0.1 per 1000 of all patients referred for constitutional chromosome abnormality detection. This group consisted of children with autism, mental retardation or congenital malformations and couples with reproductive failure.
Belonnow et al (1995) recorded a large Scandinavian experience of 50 ESACs, almost half were inv dup(15), six were small rings deriving from various autosomes, six were isochromosomes of 18p or 12p and most remainder were harmless ESACs derived from acrocentric chromosomes.In our retrospective data analysis of fifteen marker chromosomes, three were i(18)p11, two were i(15)p11, one was i(22)p11, one characterized as i(Y)p11, three were small rings, and three had low level mosaicism and two metacentric markers.
Inversion duplication 15:
Formation of inversion duplication shaped SMC an intra or interchromosomal U-type exchange of homologous chromosomes may have occurred during meiosis.
Inv dup(22)(pterq11.2)
This causes cat eye syndrome. The duplication is not always symmetrical, suggesting that the chromosome can arise from misalignment and exchange over a considerable distance.
Isochromosomes (i18p)
Isochromosome is a mirror image. Isochromosome marker which have been reported are i(5p), i(9p),i(12p),i(18p), i(21p),i(22q) and i(Xq). The classical mode of isochromosome formation is by transverse division at the centromere in a chromosome [ eg.chromosome 5 forms i(5p) and i(5q)]. U-loop formation during meiosis is also postulated.
Small or minute ring
These patients had an ESAC in the form of a small ring chromosome. This may have derived from any of the 24 chromosomes. The ESAC is regarded as harmless in a phenotypically normal individual. This category of ESAC is called the B chromosome. They are found by chance in normal individuals or at prenatal diagnosis. It may be a cause of reproductive failure if it contributes to errors of cell division during meiosis. Jafar et al (1994) reported an otherwise phenotypically normal male who presented with infertility. He had a bisatellited heterochromatic chromosome. In most spermatocytes the ESAC was in close proximity to the X-Y bivalent and this have been the cause of infertility.
Parental partial aneuploidy (mosaicism)
There were patients with presence of ESAC in mosaic state. This could not be characterized as the mosaicism was low level .
Transmission of an ESAC which contains active genetic material can cause functional partial aneuploidy and may be associated with reproductive risks if the abnormal cell line is represented in the gonads.
Mosaicism or familial mosaics are frequently seen . Majority of these harmless ESACs comprise acrocentric short arm and pericentric material. Familial ESACs are characteristically maternally transmitted, which could reflect preferential exclusion of the marker in spermatogenesis or possibly a reduced male fertility.
I(Yp)
We found one patient with isoY. This may be the cause of infertility.
Determining the origin of the chromosome material of the marker has been felicitated by molecular cytogenetic techniques, particularly by FISH. Classification of the marker chromosomes is important for phenotype-karyotype correlations, which is imperative for proper and personalized Genetic counseling. Prenatal diagnosis is offered for an ESAC in which a risk is judged.
Approximate risks of fetal abnormality following prenatal detection of an ESAC depends on the level of characterization.
|
Extra Structurally Abnormal Chromosome |
Risk |
|
I(18)p |
100% |
|
I(12)p |
100% |
|
Idic(22) |
100% |
|
Larger der(15) breaks distal to q11.2 |
95% |
|
Any non-characterized marker ,excluding i(18)p and i(12)p |
13% |
|
Small mostly C-band positive |
5% |
|
Small der(15) breaks proximal to q11.2 |
5% |
|
Der(Y) |
<5% |
|
Dots |
<5% |
|
Small bisatellited, single centromere |
<2% |
(Gardener et al)
15000blood samples were referred to Cytogenetics, Center of Medical Genetics, Sir Ganga Ram Hospital to detect constitutional chromosomal abnormalities by karyotyping. These referrals comprised of Childrenwithdysmorphicfacial features/congenitalmalformations and healthyadult whohas experienced problems with fertility or abortions.
In conclusion, characterization of the ESAC is of utmost importance. Based on the morphology of ESAC with cytogenetic and clinical data, different molecular strategies can be applied to characterize the ESAC and develop the phenotype-karyotype correlation for better and personalized Genetic Counseling and prenatal diagnosis of the future offspring.
References:
[1] B. Maurer, T. Haaf, K. Stout, N. Reissmann: Two supernumerary marker chromosomes, originating from chromosomes 6 and 11, in a child with developmental delay and craniofacial dysmorphism
[2] M. Balkan, H. Isi, A. Gedik, M. Erdemoglu and T. Budak: A small supernumerary marker chromosome, derived from chromosome 22, possibly associated with repeated spontaneous abortions
[3] Chih-Ping Chen, Chyi-Chyang Lin, Yi-Ning Su, Fuu-Jen Tsai, Schu-Rern Chern, Chen-Chi Lee, Dai-Dyi Town, Li-Feng Chen, Pei-Chen Wu, Ju-Ting Chen, Taiwan J Obstet Gynecol, Wayseen Wang: Prenatal diagnosis and molecular cytogenetic characterization of a small supernumerary marker chromosome derived from chromosome 18 and associated with a reciprocal translocation involving chromosomes 17 and 18.
[4] Jie Hu, Suneeta Madan-Khetarpal, Stephanie J. DeWard, Alvaro H. Serrano Russi, Malini Sathanoori and Urvashi Surti: Three Supernumerary Marker Chromosomes in a Patient with Developmental Delay, Mental Retardation, and Dysmorphic Features.
[5] Knight S and Flint J(2002) Mullti-Telomere FISH from: Molecular Cytogenetics: Protocols and applications . Ed: Y S. Fan. Humana Press Inc., Tolowa,
[6] N J Clouston H J. 2001. Lymphocyte culture. In: Rooney D editor. Human Cytogenetics constitutional Analysis, 3edition. Oxford New York: Oxford University Press, (p33-54).
[7] Buckton et al., 1985.M. Balkan, H. Isi, A. Gedik, M. Erdemoglu and T. Budak: A small supernumerary marker chromosome, derived from chromosome 22, possibly associated with repeated spontaneous abortions.
[8] ISCN (2009). An international System for Human Cytogenetic Nomenclature. L G Shaffer, M L Slovak, L J Campbell (eds); S Karger, Basel.
[9] Small Supernumerary Marker Chromosomes (sSMC): A Guide for Human Geneticists and Clinicians by Thomas Liehr
[10] iGenetics: A Molecular Approach (3rd Edition) by Peter J. Russell
[11] Principles of Genetics by D. Peter Snustand and Michael J. Simmons
[12] Gerson S.L. and Keagle M. B. – The Principle of Clinical Ctogenetics.
[13] Turnpenny Peter and Eliard Sian – Element of Medical Genetics
[14] Purendre Hema – Clinical Cytogenetics
[15] rarechromo.org retrieved on 16 May 2012.
[16] Tijo and Levan 1956.
[17] Lejeune 1959
[18] Ilberry et al. 1961
[19] Seabright 1971
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









