 About Authors:
About Authors:
Deepak singh1*, Arpit Dixit2, Amir khan3, Vikas singh4, Abhishek sachan4
1*Department of Clinical Research, Jamia Hamdard, Hamdard nagar, New Delhi-110062
2Business executive at Merck Pvt.ltd, Ghaziabad, India
3Business executive at Cipla Pvt.ltd, Lucknow, India
4Shri RLT Institute of Pharmaceutical Science & Technology, Etawah(UP), India
*deep_singh4u21@rediffmail.com
ABSTRACT:
The present investigations, which were primarily conducted with the aim of investigating some neuropharmacological activity of Polygonum glabrum (PG), i.e. PG has got anxiolytic activity when tested against open field exploratory behavior, where as elevated plus maze did not show any positive results. The action produced by PG was more than that of diazepam in open field exploratory behaviour. Observations confirms that PG possesses significant antidepressant activity. The observed antidepressant activity of PG was qualitatively comparable to that induced by Imipramine. Pentobarbitone induced hypnosis in mice was significant potentiated by PG.PG at 100 and 200mg/kg, reduced locomotor activity in rats.The PG seems to be little or no motor incoordination effect in mice when tested against rota-rod test.PG had significant analgesic activity which is both centrally and peripherally mediated, when tested against various analgesic models in rodents.The investigations indicates that PG has significant analgesic, anti-inflammatory, antidepressant and anxiolytic actions, some of these actions, including antidepressant and anxiolytic can be rationalized on the basis of the neurochemical data emanating from this study . The present study indicate that PG can be clinically useful not only in inflammation, pain and fever, and worm infestation but also in depression and anxiety. Clinical studies are required to confirm the above mentioned activities.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2528
INTRODUCTION :
Polygonum species are known to ancient communities as useful medicinal plants. Polygonum glabrum Willd is a perennial plant belonging to Polygonaceae family. It is commonly known as Densflower knotweed. The genus Polygonum includes 150 species (Graham 1958)8. According to geographical origin, there are different synonyms for the plant. Polygonum glabrum Willd. Asia is similar to that tropical Africa and Egypt, but the Asian species has absolutely glabrous leaves with eciliate margins. Polygonum senegalense Meisn (Polygonum glabrum Willd) is found in Senegal; the leaves are glabrous with the limb surface exuding a viscous yellow liquid , occasionally, young leaves having deciduous tomentous pubescence are found (Hutchinson &Dalziel 1963; Berhaut 1967)44. In Kenya, there are two forms of plant ; Polygonum senegalense formasenegalens, distinguished by dark green leaves with a yellow sticky oily residue covering the surface ,and Polygonum senegalenes forma albotomentosum , distinguished by the leaves covered with white hairs (Graham 1958)8. The species ofPolygonum glabrum found in India and Sudan seems morphologically comparable.
Indian Polygonum glabrum is a perennial herb growing up to a height of 150cm, distributed widely in the eastern India at altitude of 1900m, especially near liberal water source.Polygonum glabrum contains numerous compounds with documented biological activity.
Constituents that have stimulated the most interest include the sesquiterpenes, polyphenols like vannilic, syringic, p-hydro benzoic, protocatecheuic, galliccis & p-coumaric acids, kaempferol quercitrin and essential oils.
Quercetin, rhametin, quercitrin, avicularin and rutin were isolated from ethanolic extract of the leaves.(Tiwariet al;1979)9
In India the leaves of this plant are used for treatment of colic pain, fever, and for “stich in the side” (Kirtikaret al; 1975)6 while in Sudan they are used to treat roundworm and tapeworm infestations (O.Abdel-Moneim, Sudan National Council for Research 1979, personal communication). The Sudanese species has received little photochemical examination but molluscicidal properties and principals of the Kenya species have been reported (Dossajial1976: Maradufu&Ouma1978; Dossaji& Kubo 1980)10.
It is also reported to possess antiviral (Bhakuniet al; 1985)12 and antibacterial (Krishnamurthi, 1969)12 activities.
Polygonum glabrumhas been studied for anti-inflammatory (Bhupinderet al; 1987)4, analgesic, hypotensive and spasmogenic (Singh et al; 1985)11. The finding show that Polygonum glabrum extract is clinically effective as an anti-inflammatory drug and works by the mechanism of action similar to NSAIDs. Polygonum glabrum also has been researched for anthelmintic activity (Muddathiret al; 1987)13s,which showed activity especially against Hymenolepis nanavar fraternal.The above information reveals that Polygonum glabrum has got extensive pharmacological actions. The lack of viablepharmacological mechanism or the assurance of which components within the plant are critical for therapeutic effect (necessary in order to standardize formulations) . Over the centuries, societies around the world have developed their own traditions to make sense of medicinal plants and their uses. Some of those traditions and medicinal practices may seem strange and magical, others appear rational and sensible, but all of them are attempts to overcome illness and suffering with an aim to enhance the quality of life. Many of the thousands of plant species growing throughout the world have medicinal uses, containing active constituents that have a direct pharmacological action on the body. There is increasing interest in industry, academia and health sciences in medicinal plants57
MAIN CONTENT:
EXPERIMENTAL WORK
Pharmacognostical studies:
• Collection and authentication of plant
• Drying and size reduction
Extraction
• Isolation & Purification
• TLC
• HPLC
Phytochemical studies:
• Preliminary Phytochemical studies
Pharmacological studies
• Antidepressant activity
Pharmacognostical studies:
• Collection:
The leaves of Polygonum glabrum Willd were collecte during month of August from the Ayurvedic Garden of National Botanical Research Institute, Lucknow. Authentication was performed by joshi et al., (1987)23.
• Drying and size reduction:
The leaves of Polygonum glabrum Willd were dried in the shade for about a week followed by drying at 30ºc-40ºc in oven for a day. The leaves were then grinded to coarse powder in Mortar and Pestle. The powdered materials was passed from sieve no. 20(#). Finally the powder is used for extraction.
Extraction:9,24,27
Extraction was a process where by the desired constituents of a plant are removed using a solvent. The primary ways for extraction of organic molecules of interest to biologists and medical investigators involve breaking open the cells. the classical chemical procedure for obtaining organic constituents from dried plant tissue (heart wood , dried seeds, root , leaf) was to continuously extract powder material in a soxhlet apparatus with a range of solvents , starting in tern with ether, petroleum and chloroform (to separates lipids and terpenoids ) and then using alcohol and ethyl acetate (for more polar compounds ). This method was useful when working on the gram scale.
Extraction Methods:
These are the extraction methods which were used in phytochemistry.
Maceration: This method involves shocking and agitating the solvent and plant materials together. The solvent was then drained off. Remaining miscella was removed from the plant material through pressing or centrifuging. This method does not totally extract the active ingredients from the plant materials.
Percolation: With this method, the plant material is moistened with solvent and allowed to swell before being placed in one of a series of percolation chambers. The material is repeatedly rinsed with solvents until all the active ingredients have been removed. A solvent is reused until it is saturated. New solvent is used on plant material that is almost completely exhausted, and then reused on subsequently less exhausted batches. This method is more is more effective in obtaining active ingredients than the maceration technique.
Soxhlet extraction:
The use of commercially available soxhlet extractor was a convenient way to prepare crude plant extracts. This procedure was used mainly with pure solvents, although some although some authors have reported the use of binary or ternary solvent mixtures. Mixed solvents suffer the inconvenience that individual components may distill at different temperature, so that the resulting mixture in the chamber containing the drug was enriched in the solvent of lower boiling point. Thus actual solvent proportions in the extracting chamber differ from that originally used in the collector, and this fact may introduce errors when typing to reproduce the experiment using other extraction methods. The main advantage of extraction using a soxhlet apparatus was that it is an automatic, continuous method that does not require further manipulation other than concentration of the extractive and saves solvents by recycling it over the sample. Moreover, this method is not time-consuming, since for a standard sized sample (500g), the extraction time is less than 24 h. an obvious disadvantage is that the extractives were heated during the period of extraction at the boiling point of the solvent employed and thermally labile compounds such as carotenoids may hydrolyze or decompose.
Infusion and decoction:
Infusion and decoction are simple methods for extraction with water. In the latter, in the former case, hot or cold water is added to the milled drug; in the letter, the sample is boiled for about 15 min. in water. Extraction with water as the role solvent is seldom used for plant material, although some plant constituents are water-soluble, such as carbohydrates, flavonoid polyglycosides, quaternary alkaloids, saponins and tannins. As an example
•Extraction of Polygonum glabrum leaves
Dried leaves of Polygonum glabrum (about 100gms) was extracted with 300ml methanol (90%) in a Soxhlet apparatus for 72hrs. After extraction, the solvent was filtered under reduced pressure.
•Isolation and purification:
After extraction of natural products they are subjected to isolation and purification to ascertain what the natural product is and to carry out sufficient experimental work necessary to biologically characterize or profile the compound. The techniques available include, but are not limited to, solid-phase extraction, high-performance liquid chromatography (HPLC), gradient high-performance liquid chromatography, thin-layer chromatography (TLC), column chromatography, paper chromatography, etc. Chromatography is the method of choice in handling the problem of isolation of a compound of interest from a complex natural mixture 11. Therefore, the chromatographic methods commonly used during an isolation work are briefly described.
Chromatography is a separative technique that has arisen from the chemists’ interest to be able to separate a mixture of compounds into its constituents. The name chromatography was firstly used in 1906 by a botanist called Tswett; who worked to separate colored plant pigments. Chromatography means color-writing in Greek. Tswett made his experiments by allowing a solution of mixed pigments to pass through a column of crushed chalk and than he observed the separated color zones on the column. The international Union of Pure and Applied Chemistry (IUPAC) defined chromatography as follows:
Methods used primarily for the separation of components of a sample, in which components are distributed between two phases, one of which is stationary while other moves. The stationary phase may be a solid, or a liquid supported on a solid, or a gel. The stationary phase may be packed in a column, spread as a layer, or distributed as a film, etc. In these definitions “chromatographic bed” is used as a general term to denote any of the forms in which the stationary phase may be used. The mobile phase may be a gas or a liquid (Sewell, 1987).
Thin layer chromatography:
Thin layer chromatography is a rapid, simple, versatile, sensitive and inexpensive analytical technique for the separation molecules. The stationary phase in TLC is a thin layer of adsorbent that is spread uniformly over a plate. The plate can be prepared by spreading the adsorbent on glass in the lab or commercial pre-coated plates can be used. . The major advantage of TLC is the disposable nature of the plates. Samples do not have to undergo the extensive clean-up steps required for HPLC. The other major advantage is the ability to detect a wide range of compounds cheaply, using very reactive reagents (iodine vapors, sulfuric acid) or indicators. Nondestructive detection (fluorescent indicators in the plates, examination under a UV lamp) also means that purified samples can be scraped off the plate and be analyzed by other techniques. Thin layer chromatography has traditionally been considered as a pilot technique for column chromatography and high performance liquid chromatography. TLC has also been considered to be a suitable pilot technique for solvent or sorbent optimization because it is more flexible, more rapid and cheaper than column techniques8.
Chromatographic solvent "polarity"-
There is four major intermolecular interactions between sample and solvent molecules in liquid chromatography, dispersion, dipole, hydrogen-bonding, and dielectric. Dispersion interactions are the attraction between each pair of adjacent molecules, and are stronger for sample and solvent molecules with large refractive indices. Strong dipole interactions occur when both sample and solvent have permanent dipole moments that are aligned. Strong hydrogen-bonding interactions occur between proton donors and proton acceptors. Dielectric interactions favor the dissolution of ionic molecules in polar solvents. The total interaction of the solvent and sample is the sum of the four interactions. The total interaction for a sample or solvent molecule in all four ways is known as the "polarity" of the molecule. Polar solvents dissolve polar molecules. For normal phase partition chromatography, solvent strength increases with solvent polarity, whereas solvent strength decreases with increasing polarity in reverse-phase systems.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Phytochemical studies:
• Preliminary Phytochemical studies2, 25
Preliminary test was carryout for the presence/absence of phytoconstituents like Alkaloids, Glycosides, Carbohydrates, Flavonoids, Reducing sugars, Saponins, Sterols, Terpenes, Tannins.
Choice of solvents:-
According to phytochemical studies, methanolic extract contains more chemical constituents than other solvent extract. So Methanol was selected as choice of solvent for extraction of dried leaves of Polygonum glabrum.
Solvent purification:-
The various solvents there may be admixed with any other substances, so it needs purification before use. Here the solvents are subjected to simple distillation under atmospheric pressure.
A description of method was adopt for performing the tests are summarized below.
1. Alkaloids
(A) With Mayer’s reagent:-
The methanolic extract was treated with few drops of dilute 2N HCl and 0.5ml of Mayer’s reagent, white ppt. obtained
(B) With Wagner’s reagent:-
The methanolic extract was treated with few drops of dilute 2N HCl and 0.5ml Wagner’s reagent, brown flocculent ppt. was obtained.
(C) With Dragendroff’s reagent:-
The methanolic extract was treated with few drops of dilute 2N HCl and 0.5ml Dragendroff’s reagent ,brown ppt. was obtained.
2. Phenols
(A) With neutral FeCl3:-
The extract was treated with neutral FeCl3, formation of blue, green, or red color, indicates presence of phenols.
(B) With Ferrous sulphate and Sodium potassium Tartarate:-
The reagent was prepared by mixing 0.1% Ferrous sulphate solution and 0.5% Sodium potassium Tartarate solution. Few drops of reagent was added to a little of the residue, blue, green, violet, or red colouration was produced. Phenols containing two hydroxyl groups to each other give this test.
3. Glycosides
(A). Molisch’s test:-
To a small portion of the extract and 2 drops of 10% solution of α-Napthol in chloroform. Carefully added 1 drop of concentrated H2SO4 from a finely pointed pipette so that it form a separate layer below the water. A violet ring formed at junction of the two layers. On mixing the layers by shaking the test tube in a stream of cold water, a deep purple solution results which on dilution with cold water yields a violet ppt. On shaking and adding a small quantity of suspension to an excess of conc. Ammonia, the color is changed to dull brown.
(B).With Concentrated H2SO4 :-
Extract containing glycosides gives intense colours when treated with cold concentrated H2SO4 .
4. Flavonoids
(A). Shinoda’s test:-
An alcoholic extract of leaves was treated with Magnesium (dust) and concentrated HCl, the appearance of pink tomato colour indicates the presence of flavonoids.
(B).Dissolve sample in 10% HCl and add Zink (dust), pink colour effervescence indicates the presence of flavanones and flavanols.
(C).When treated with neutral lead acetate gives yellow, orange, red, or brick colour, indicates the presence of flavanoids.
(D).Aques extract dissolved in H2SO4 to give intensely yellow solution.
5. Terpenoids and Steroids
The terpenoids have a common biosynthetic origin. The terpenoids includes
Essentiol oils
Diterpenoids and Gibberlines
Triterpenoids and Steroids
Caretonoids etc.
Lebermann-Burchard reaction
The extract was treated with acetic anhydride and concentrated H2SO4, gives purple red color.
6. Test for Reducing Sugars
(A).Benedict’s test:-
Take 5ml of reagent in test tube, add 8 drops of extract. Placed in boiling water bath for 5min. A green, orange-red ppt. gives a semi quantitative estimation of reducing sugars.
(B). Fehling’s test for Reducing Sugars:-
Take 2ml of extract, added equal volume Fehling A and Fehling B mixture, placed in boiling water bath for 5min. A red ppt. was obtained.
7. Saponins
(A) Add some water to sample and shake vigorously formation of stable froth with honeycomb structure indicates the presence of saponins.
(B).To an aqueous solution of extract, add solution of lead acetate, formation of white ppt. indicates the presence of saponins.
8. Tannins
(A) A small portion of extract was treated with 5% Ferric Chloride solution. The production of green, to blue color was taken as positive test for tannins.
(B). A creamy ppt. with lead acetate was considered positive test for tannins.
Data for Qualitative Phytochemical studies
|
1. |
Alkaloids 1-Dragendroff’s test 2- Wagner’s test 3- Mayer’s test 4- Hager’s test |
+ -- + + |
|
2. |
Phenol 1- With neutral FeCl3 2- With FeSO4 & Sod. Pot.Tartarate |
-- + |
|
3. |
Glycoside 1- Molisch test 2-With Conc.H2S04 |
-- -- |
|
4. |
Flavonoids 1- Sinoda test |
+ |
|
5. |
Terpenoide 1-Libermann Burchared test |
+ |
|
6. |
Reducing Sugar 1-Benedict test 2-Fehling test |
-- -- |
|
7. |
1-Saponine test |
+ |
|
8. |
Tannin |
-- |
Pharmacological studies
Animals: Adult Charles Foster albino rats (200±20g), of either sex, were obtained from the Animal house of S.R.L.T, and were randomly distributed into diffrent experimental graph. The rats will be house in group of six in polypropylene cages at an ambient temperature of 250C ± 10Cand45-55%RH, with 12:12 h light/dark cycle. Animal were provided with commercial food pallets and water ad libitum unless stated otherwise. Experiments were conducted between 9.00 and 14.00 h. Animal were acclimatized for at least one week before using them for experiments and exposed only once to every experiment. “Principals of laboratory animal care” (NIH publication number 85-23, revised 1985)28 guidelines were followed.
Drug treatments:
The methanolic extract of Polygonum glabrum were protected from light and stored under refrigeration at 0-40C until its use. Extract was solubilized in distilled waterbefore experimental studies, and administered either orally or intraperitoneally at different dose levels for three consecutive days. Control animals were treated with distilled water 10ml/kg. Standard drugs were used in each set of experiment accordingly and were administered either orally or intraperitoneally to rodents 1h or 30min before experiments for comparison respectively. Experiments were conducted on day 3, 1hr after the last drug administration.
Statistical analysis: The data were expressed as means ±SEM for each treatment group. Statistical significance between groups was performed by the application of analyses of variance ANOVA followed by Dunnett’s test, p values less than 0.05 (p < 0.05) were used as the significant level.
Drug and Chemicals for Screening Methods: The following drug and chemical used and all the reagent and chemicals were of analytical grade.
• Phenobarbitone (Sigma,St,Louis,USA)(20MG/KG,i.p.) was used as standard anticonvulsant agent.
• Dopamine (DA),Norepinephrine(NE), Serotonin(5-HT),and 5-hydroxy indole acetic acid (5-HIAA) was procure from Sigma,St Louis, USA.
• n-Butanol(fluorometric grade) use without further purification.
• n-heptane was wash with one-fifth volume 1N NaOH,then with 1N HCl.
• Diethyl ether was wash with a saturated solution of FeSO4 to remove accumulated peroxides and subsequently with distilled to remove the FeSO4.
• Ethylene diamine was redistill and store in a dark bottle in the cold.
• Acetate buffer (2M,ph 6.8): 2N acetic acid to be adjusted with 2N NaOH to a ph of 6.8.
• Iodine Solution (0.1N): Iodine (3.175gm) to be dissolve in distilled water containing 12.5gmNaI, diluted to 250 ml, and store in a dark bottle in the cold.
• Sodium thiosulfate,(0.1N): Na2S2O3.5H2O(6.2gm) to be dissolve in distilled water, make to 250 ml, and were stored in a dark bottle in the cold.
• EDTA (10%w/v) Ethylene diamine tetra acetate, disodium salt(10gm) to be dissolve in distilled water with heading and dilute to 100ml.
• Alkaline Sulphite/ EDTA Solution: Na2SO3.7H2O(12.6gm) to be dissolve in 25ml of 10% EDTA and was dilute to 250ml with 5N NaOH.
• Alkaline ascorbic acid/ethylenediamine solution: Immediately before use, ascorbic acid (200mg) to be dissolve in 2.5ml 0.01n HCl,and was added to 22.5ml 10N NaOH containing 0.5ml redistilled ethylenediamine Since the solution was very viscous, It was necessary to mix it thoroughly.
• HCl/acetic acid reagent: Concectrated HCl and glacial acetic acid were mixed in a 1:1 ratio.
• Ascorbic acid solution: immediately before use,100mg ascorbic acid was dissolved in 10ml 0.01N HCl.
Antidepressant activity:
The chemicals /Plant extract which reduces the mental depression which characterized by feelings of intense sadness and despair, mental slowing and loss of concentration, pessimistic worry, lack of pleasure, self-deprecation, and variable agitation or hostilitymay called as Antidepressant Drugs.
Mechanism of Action:- Antidepressants enhance the biological activity of monoamine neurotransmitters in the CNS and that anti-adrenergic compounds may induce depression.
The Antidepressant drugs have effects on cortical, limbic, hypothalamic, and brainstem mechanisms that are of fundamental importance in the regulation of arousal, consciousness, affect, and autonomic functions30.
Apparatus: Graduated glass box (45×20cm) containing 38 cm water
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Behavioral despair test: (Willner, et, al. 1984)32
The rats were placed in cylinder (45×20cm) containing 38cm water (25±2?c), so that the rat could not touch the bottom of the cylinder with its hind limb or tail, or climb over the edge of the chamber. Two swim sessions were conducted, an initial 15min pretest, followed by a 5min test 24h leter.
Drugs were administered after pretest. The period of immobility (remained floating in water, without struggling and making only those movements necessary to keep its head above water) during 5 min test period noted.
Tail suspension test in mice: (Chermat, et, al.1986)33 (Steru et, al.1985)42
The male mice (20-25gm) were housed in plastic cage for at least 10 days prior to testing in a 12 h light cycle with food and water freely available. Animals were transported from the housing room to the testing area in their own cages and allowed to adapt to the new environment for 1h before testing. Groups of 10 animals were treated with test compound or the vehicle i.p. 30min prior to testing. For the test the mice were suspended on the edge of shelf 58cm above the table top by adhesive tape placed approximately 1cm from the tip of the tail . The duration of immobility was recorded for a period of 5min. Mice was considered immobile when they hung passively and completely motionless for at least 1min
Learned helplessness test in rats : (Chabner, et.al.1996)34
Learned helplessness was produced in male Sprague-Dawley rats (300gm)by exposure to electric shock (0.7mA) for 1h on a schedule of 10s of shock/min. The apparatus was a 30×45×30cm box with grid floor. At a height of 20cm above the floor ,a platform (7.5×7.5cm)can be inserted through one side wall to allow a jump-up escape response. The platform is not available during training. After the appropriate treatment, the animals were tested for acquisition of a jump-up escape in the same apparatus. At the beginning of trail the platform is pushed in to the box and a 0.4mA shock initiated. Shock was terminated in 10s if the animal has not escaped on to the platform by this time. If an escape response occurred, the animal is allowed to remain on the platform for the duration of 10s, then returned to the grid floor. Ten such trails with an inter-trail interval of 20s are given. In a naïve control group of rates, this training resulted in 80% acquiring helplessness behavior. Drugs were given before the training and test period.
LITERATURE AND TABLES :
The name Polygonum derived from the Greek word poly “many,” and gonu, “knee or joint,” hence “many joints” because of the thickened joints on the stem. The species name glabrum is based on the smooth or hairless appearance of the leaves. Polygonum glabrum is known as Denseflower knotweed due to its morphological appearance of the flower and stem.
Polygonum glabrumis the perennial plant belonging to the family Polygonacy. The genus Polygonum encompasses 150 species, of which 79 are known to occur in India (Hooker, 1985)15. Polygonum glabrum is distributed in Asia, Africa, and North America. Indian Polygonum glabrum is perennial herb growing up to a height of 150cm distributed mainly in the eastern area at altitude of up to 1900m, especially near liberal water source.
A good number of species of Polygonum have been used traditionally from a long time for a number of ailments in the indigenous system of medicine. The medicinal properties attributed to the species of Polygonum are demulcent and pectoral, astringent and tonic, diuretic, emetic, purgative, febrifuge, vesicant vulnerary, insecticide, and anthelmentic (Kirtikaret.al., 1975)6. Besidesit also possess antiviral (Bhakuniet.al., 1969)12 and antibacterial (Krishnamurthi, 1969)12 properties.
Ethno-medicinal investigation
Polygonum glabrumhas been studied for anti-inflammatory and anthelmintic activity. Recent interest has focus on its C.N.S. activity. Polygonum glabrum also has been investigated for hypotensive and spsmogenic activity (Singh et.al., 1985)11. Polygonum glabrum contains several compounds of biological interest, including the sesquiterpenes and broad spectrum of flavonoids.
Anti-inflammatory activity
Anti-inflammatory studies were conducted on a hot water decoction and on an ethanol extract of the stem of Polygonum glabrum (Bhupinderet.al., 1987)4. It has been shown a significant anti-inflammatory activity against carrageenan-induced pedal edema in rats.
Polygonum glabrumwas also studied in another models representing acute, sub-acute and chronic inflammation. It was observed to be consistently effective in suppressing the exudates and granuloma formation in the granuloma pouch test, the formaldehyde-induces chronic arthritic reaction and immunologically-meted adjuvant- induces poly arthritis syndrome. The latter is accepted as a models having chronicity and immunological basis, two important feature of human rheumatoid arthritis (Billingham and Davies,1979)16, there this procedure is probably the most important and valid screening method. Significantly, 18h data on the primary reaction in the injected limbs also been reported to be a more specific and reliable model of acute inflammation than the commonly employed carrageenan paw edema test (Sofia et al., 1975)17. The effects of Polygonum glabrum were approximately similar in the models after i.p as well as p.o administration, lending greater validity to the anti-inflammatory activity of Polygonum glabrum .In addition Polygonum glabrum was equally active against the acute 4h reaction to formaldehyde.
Polygonum glabrum has been observed to be an effective anti-inflammatory agent against three models of acute inflammatory reaction and three models of sub acute or chronic inflammation. It was observed that PG was relatively less potent than phenylbutazone in most acute models while it was usually more potent in the sub acute or chronic models. This pattern was observed both after oral and i.p. administration although, dose for dose, Polygonumglabrum was less effective orally indicating poorer bioavailability of the active principle due to either to partial absorption or to a first pass metabolism by the liver. (Prado et al, 1990)26
Anthelmintic Property:-
A pure anthelmintic substance (PGA) has been isolated from the leaf of Polygonum glabrum willd. (Muddathiret al.,1987)13. The antiparasitic in-vitro activity of several fractions isolated from the plant has been examined comparatively with that of PGA. PGA also showed molluscicidal activity against Biomphalaria glabrata and Limneatruncatula Mull.
PGA had highly significant taenicidal activity in-vitro, whereas it had weaker trematodicidal and amoebicidal activities. The anthelmintic activity was mainly studied against Hymenolepis nana var. fraternal of the white mouse.
The in-vitro screening for anti-parasitic activity of divers fraction isolated from the leaf of, Polygonum glabrum confirmed the properties of PGA the anthelmintic activity against Hmenolepis nana var. fraternal being observed at LD=2×10-3 mg/ml which is ten times lower then that of crude extract.
The trematodicidal activity of PGA against fasciola was observed at LD= 6×10?1 mg/ml while the crude extract was inactive.
The amoebicidal activity of PGA against Entamoeba histolytica was observed at MID =5×10-1 mg/ml.
The in-vivo anthelmintic activity of PGA was assayed against Hymenolepis nana ver.fraternal at 200-600 mg/kg and gave no positively significant results; however it has been observed that a good tolerance of PGA in white mice at dose=400mg/kg.
PGA and crude extract of PG were molluscicidal against Biomphalaria glabrata and Limnea truncatula Mull, while El-Tohami (1979)21found aqueous extract from the leaves of same plant not to be molluscicidal against Biomphalaria pfeifferi and Bulinus truncatus (from Sudanese breeding), when assayed at 250ppm. Crude fraction gave 100% mortality of B.glabrata 40% mortality of Limnea truncatula, after 24h exposure at 100ppm. PGA gave 100% mortality of P.glabrata at 1-0.5ppm and 100% mortality of Limnea truncatula at 2-1ppm, after 24h exposure.
Other effects
P.glabrumhas been reported to have number of additional effects. In one study the crude aqueous extract has been produced hypotention and reduce respiration rate. These effects could not be modified by atropinisation (Singh et.al., 1985)11. Whereas leaves extract has shown falling effect in b.p. and caused increase in the rate and volume of respiration.
Both stem and leaves portion individually produced a spasmogenic response in albino rat’s and rabbit’s intestine. The spasmogenic activity of aqueous leaves extract inhibited completely by pheniramine and atropine which shows that this response is mediated through cholinergic and histaminergic system (Singh et.al., 1985)11.
Toxicology
The toxicological studies showed that P.glabrum appears to be possess a reasonable margin of safety (Bhupinderet.al., 1987)13.P.glabrum up to 2g/kg p.o. did not cause any death in the mice.
The LD50 after a series of i.p. doses was 10mg/kg approximately by Karber’s method (1931)18 and 760mg/kg (±125mg/kg S.E.) by the graphic method of Miller and Tainter (1944)19. Polygonum glabrum administration in dose up to 50mg/kg i.p. and 200mg/kg p.o. once did not cause any death in the rats up to 30days.
Phytochemical investigation
Phytochemistry:
Phytochemistry is in the strict sense of the word the study of phytochemicals. These are chemicals derived from plants. In a narrower sense the terms are often used to describe the large number of secondary metabolic compounds found in plants. Many of these are known to provide protection against insect attacks and plant diseases. They also exhibit a number of protective functions for human consumers Techniques commonly using in the field of Phytochemistry are extraction, isolation and structural elucidation (MS,1Dand 2D NMR) of natural products, as well as various chromatography techniques (MPLC, HPLC, LC-MS).
The title of a book on hallucinogens by Efronet al.‚ namely, "Ethnopharmacologic Search for Drugs", where it was defined. (Wada et al, 1989)20
Terms and concept: The term “Ethnopharmacology” was first used in 1967 as “The inter diexploration of biologically active agents traditionally employed or observed by man”.
Shortly before the start of the 20th century American botanist William Hershberger coined another frequently used term “Ethnobotany”. Ethnobotany is a multidisciplinary science defined as the interaction between plants and people.
In order to highlight the role pharmacy as a profession can play in the development of traditional ethnophamacopoeias, recently the concept of "Ethnopharmacy” was proposed by Heinrich, it has been defined as the interdisciplinary science that deals with the study of the pharmaceutical means, considered in relation to the cultural determinants that characterizes the uses of these means in a given human group. It involves studies of the identification, classification and cognitive categorization of the natural material from which the remedy will be produced, which encompasses not only botanical and pharmacological but also phytochemicals, galenicals, drug delivery, toxicological, clinical, pharmacy practice/anthropological, historical and other aspects of research on medicinal plants in popular and traditional medical systems.4
Phytochemistry is widely used in the field of Chinese medicine especially in the field of herbal medicine5.
Phytochemical technique mainly applies to the quality control of Chinese medicine or herbal medicine of various chemical components, such as saponins, alkaloids, volatile oils, flavonoids and anthraquinones. In the development of rapid and reproducible analytical techniques, the combination of HPLC with different detectors, such as diode array detector (DAD), refractive index detector (RID), evaporative light scattering detector (ELSD) and mass spectrometric detector(MSD), has been widely developed 5.
In most cases, biologically active compounds in Chinese medicine or herbal medicine have not been determined. Therefore, it is important to use the phytochemicals methods to screen and analyze bioactive components, not only for the quality control of crude drugs, but also for the elucidation of their therapeutic mechanisms. Modern pharmacological studies indicate that binding to receptors or ion channels on cell membrane is the first step of some drug actions. A new method in Phytochemistry; biochromatography, has been developed. This method combines human red celll membrane extraction and high performance liquid chromatography to screen potential active components in Chinese medicine. Phytochemicals is plant or fruit derived chemical compounds. "Phytonutrients" refer to phytochemicals or compounds that come from edible plant5.
Polygonumcontains various compounds with documented biological activity2.
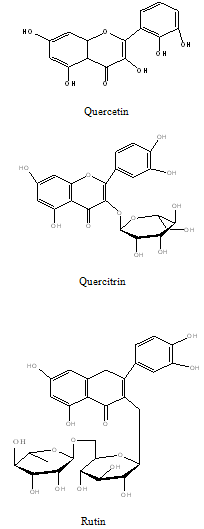
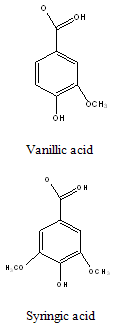
Constituents that have stimulated the most interest includes sesquiterpenes , 2,3-dihydroxyisodrimeninol and their diesters, a broad range of flavonoids including quercetin, rhamnetin, quercitrin, avicularin, and rutin, the polyphenols like vanillic, syringic, p-hydroxybenzoic, protocatecheuic, gallic and cis-and trans p-coumaric acids and essential oils.
The following major groups of bioactive constituents will summaries the constituents of Polygonumglabrum.
Sesquiterpenes: The sesquiterpenes present in Polygonumglabrum include,3-dihydroxyisodrimeninol and their diesters (Wada et al, 1989)20 1 are given below.
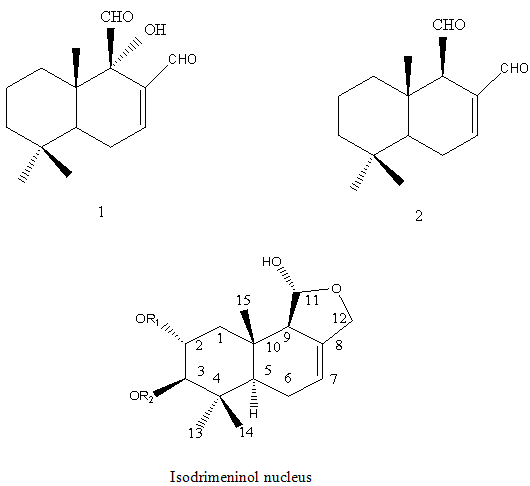
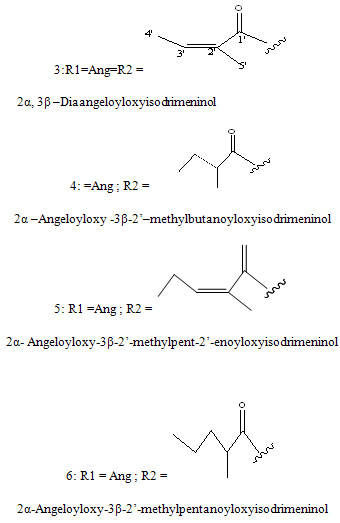
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Flavonoids: Flavonoids present in the PG include, flavonolglycoside, Viz., quercetin, rhamnetin, quercitrin, avicularin and rutin (Tiwari et al., 1979)21.
Polyphenols: Like Vanillic, syringic, p-hydroxybenzoic, protocatecheuic, gallic and cis- and trans –p-coumaric acids (Adinarayana et al., 1980)22.
Allied species
Polygonum glabrum is a perennial plant belonging to the family Polygonaceae. The genus Polygonum encompasses 150 species, of which 79 are known to occur in India (Hooker, 1885)15.
Polygonum hydropiper, or punctalum water pepper, smart weed, fifty three species of the genus of this is a biting, pungent diuretic, inflaming the tongue and skin, when applied to them. It is vermifuger, and highly stimulant. Infused in urine, it is much used gravel. It is said to cure ulcers in the mouth, toothache. The ashes makes a soap, which cutler says has been a specific for the cure of stone in the bladder. A tea of the plant is good in coughs &colds. Cattle will not touch the plant. Snake avoid it, and it kills fish. An infusion of it is a powerful promoter of urine
Pharmacological investigation
* Muddathiret al., (1987)13 isolated a pure anthelmintic substance (PGA) from the leaf of Polygonum glabrum Willd.. PGA also showed molluscicidal activity against Biomphalaria glabrata andLimnea truncatula Mull.
* Singh et.al. (1985)14 reported crude aqueous extract of Polygonum glabrum will produced hypotension and reduce respiration rate. These effects could not be modified by atropinisation whereas leaves extract has shown falling effect in B.P. and caused increase in the rate and volume of respiration.
* Bhupinderet al., (1987)14 studies on an ethanol extract of the stem of Polygonum glabrum and reported a significant anti-inflammatory activity against carrageenan-induced pedal edema in rats.
It was observed that Polygonum glabrum was relatively less potent than phenylbutazone in most acute models while it was usually more potent in the sub-acute or chronic models. This pattern was observed both after oral and i.p. administration although, dose for dose, Polygonum glabrum was less effective orally indicating poorer bioavailability of the active principle due either to partial absorption or to a first pass metabolism by the liver.
Antidepressant activity:
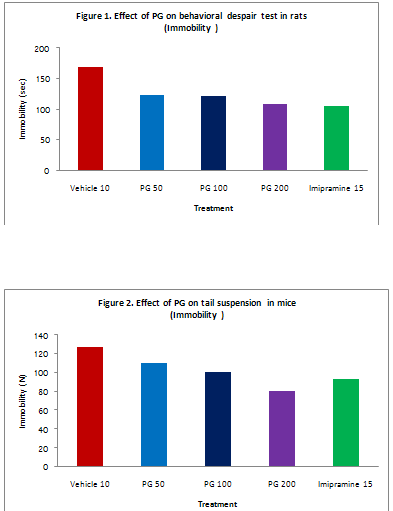
Behavioral despair test: In the initial experiment acute administration of PG extract did not reveal any antidepressant like effect in this test. Repeated oral administration of PG extract for three consecutive days reduced the immobility time in rats dose dependently. Imipramine also showed similar activity and the effect were comparable to that of higher dose of PG extract. Table 1 and figure 1.
Table 1:Effect of PG on behavioral despair test in rats
|
Treatment
|
Dose (mg/kg)
|
Duration of immobility (sec) |
|
D. water |
10ml/kg |
168.50±10.73 |
|
PG |
50 |
123.33±06.78** |
|
PG |
100 |
121.83±08.87** |
|
PG |
200 |
108.17±07.13*** |
|
Imipramine |
15 |
104.67±06.77*** |
Values are expressed as mean ± S.E.M.(n=6)
** P<0.01vs.Control.
***P<0.001vs.Control.
Tail suspension test in mice:
PG extract induced a significant and dose dependent decrease in immobility time in tail suspension test. This effect is regarded as indicative for antidepressant activity. Imipramine also showed significant antidepressant activity and the effect were comparable to that of PG. The results are shown in Table 2 and figure 2.
Table 2:Effect of PG on tail suspension test in mice
|
Treatment |
Dose (mg/kg) |
Duration of immobility (sec) |
|
D. water |
10ml/kg |
126.80±08.64 |
|
PG |
50 |
109.60±06.69 |
|
PG |
100 |
100.50±06.07* |
|
PG |
200 |
800.00±08.76** |
|
Imipramine |
15 |
93.30±05.12** |
Values are expressed as mean ± S.E.M.(n=10)
* P<0.05vs.Control.
**P<0.01vs.Control.
FIGURES :
RESULTS AND DISCUSSION :
Total yield of Polygonum glabrum extract= 1.935gms.
Rf for TLC = 60%
Rf for HPLC= 40%
DISCUSSION
Antidepressant activity
The results of present study clearly demonstrate significant antidepressant activity of extract of PG as assessed by the behavioraldespair , tail suspension tests in rats and mice. Behavioral despair was proposed as a model to test for antidepressant activity (Porsolt et al.,1977,1978)48, 49.
It was suggested that mice or rats forced to swim in a restricted space from which they cannot escape, are induced to a characteristic behavior of immobility. This behavior reflects a state of despair which can reduced by several agents which are therapeutically effective in human depression (Vogel and Vogel, 1997)50. The “tail suspension test” has been described by Steruet al., (1985) as a facial means of evaluating potential antidepressants. The immobility displayed by rodents when subjected to an unavoidable and inescapable stress has been hypothesized to reflect behavioral despair which in turn may reflect depressive disorders in humans.
Clinically effective antidepressants reduce the immobility that mice display after active and unsuccessful attempts to escape when suspended by the tail (Vogel and Vogel, 1997)50. The extract at oral doses from 50 to 200mg/kg, for 3 days significantly decreased the duration of immobility in the tail suspension test and the forced swimming test in mice and rats respectively. The behavioral effect of PG at the dose of 200mg/kg was more potent than that of Imipramine by tail suspension test.
It has been pointed out that although the majority of world`s health care services use herbal medicines (Jonas, 1997)51, the wide acceptance and rational use of such botanical medicines is possible only when the active constituents and their modes of actions are known. Taken together, the results obtained lead us to conclude that PG appears to have a certain antidepressant activity in mouse and rats models. All of these characteristics provide promise for further studies and developments of Polygonum for new antidepressants.
CONCLUSION:
The present investigations, which were primarily conducted with the aim of investigating some neuropharmacological profile of activity of Polygonum gslabrum (PG), can be summarized as follows with relevant conclusions.
• PG has got anxiolytic activity when tested against open field exploratory behavior, where as elevated plus maze did not show any positive results. The action produced by PG was more than that of diazepam in open field exploratory behavior.
• Observations confirms that PG possesses significant antidepressant activity. The observed antidepressant activity of PG was qualitatively comparable to that induced by Imipramine.
• Pentobarbitone induced hypnosis in mice was significant potentiated by PG.
• PG at 100 and 200mg/kg, reduced locomotor activity in rats.
• The PG seems to be little or no motor incoordination effect in mice when tested against rota-rod test.
• PG had significant analgesic activity which is both centrally and peripherally mediated, when tested against various analgesic models in rodents.
The investigations indicates that PG has significant analgesic, anti-inflammatory, antidepressant and anxiolytic actions, some of these actions, including antidepressant and anxiolytic can be rationalized on the basis of the neurochemical data emanating from this study . The present study indicate that PG can be clinically useful not only in inflammation, pain and fever, and worm infestation but also in depression and anxiety. Clinical studies are required to confirm the above mentioned activities.
REFERENCES:
1. Patwartha, Bhusan Aungenomics,2003. Integration for customized medicine., Indian Journal of Natural Product. 19,16
2. Kokate., C.K. Purohit A.P., Gokhale. S.B., Pharmacognosy, Nirali Prakashan, 4th Edition New Delhi 2002 :104-113
3. Sharma,S; 1995, The System of Ayurveda, Reprint 2001,Low Price Publication,Delhi, 145
4. Bhupinder, S., Panday V.B., Joshi, V.K., & Gambhir, S.S.(1987) Anti-inflammatory studies on Polygonum glabrum. Journal of Ethnopharmacology 19:257-259
5. Clark,A; 1996 Pharma Research 13,256
6. Krtikar,K.P. Basu, B.D., 1975. Indian Medicinal Plant, 2nd edition, Periodical expert, New Delhi. 887
7. James E. Robber, Varroe. Tyler, 2002. Herbes of Choice . 4-7
8. Graham, R.A., (1958) in: Crown Agents for overseas Govt. & Administration (eds) Polygonaceae in Flora of Tropical East Africa, London., 11-25
9. Tiwari, K.P., Kumar, P.,& Masood, M., (1979). LVI. OF Chem. Abart., Journal of Indian Chemical Society 92:177470d
10. Dossaji, S.F., Kairu, M.G., Gondwe, & Ouma, J.H., (1976) Lloydia 40 (2):220-223
11. Singh P.N., Sinha P. Prakash D. Kumar A. and Wahi S.P.,Priliminary evaluation of Polygonum glabrum, 22 (5), 242-246
12. Bhakuni, D.S. Dhar M.L. Dhar M.M., Dhawan B.N.and Mehrotra B.N. (1969) Screening of Indian Plants for Biological activity Part..Indian Journal of Experimental Biology 35(6),565-575
13. Muddtahir,A.K.., Balansard, G., Timon-David, P., Babadjamian, A.A., Yagoub, A.K. & Julien, M.j. (1987). Anthelmintic property of on Polygonum glabrum Journal of Pharmacology 39: 7182
14. Link site http//plants.usda.gov/index.html
15. Hooker,J.D. (1885) Flora of British India, L. Reeve & company Ltd. Ashford, Kent 5:34
16. Billingham,M.E.J. &Davies, G.E. (1979). Experimental models of arthritis in animals as screening tests for drugs to treat arthritis in man. Hand Book of Experimental Pharmacology.50(2): 108-144
17. Sofia, R.D., Rnobloch,C.L., & Vassar, B.H. (1975). Inhibition of primary lesion of adjuvant-induced polyarthritis in rats (18 hrs. arthritis test) for specific detection of clinically effective anti-arthritis drugs Journal of Pharmacology and Experimental Therapeutics 193:918-931
18. Karber, G.(1931) Archive fur Experimentelle Pathologic and Pharmacology, 162, 480 (cited in Turner, R.A. (1965) Screening Methods in Pharmacology. Acadmic Press, New York, pp 6068
19. Tainter R.C.,(1944) Pharmacological charactrisation of a novel non benzodiazepines selective anxiolytic. Japanese Journal of Pharmacology 49: 337-349
20. Wada,T., Nakajima, R., Kurihara,E., Narumi,S.,Masuoka,Y., Goto, G.,Saji, Y.,& Fukuda, N.,(1989) Pharmacological characterization of a novel non-benzodiazepines selective anxiolytic. . Japanese Journal of Pharmacology 49: 337-349.
21. Tiwari, K.P., Kumar, P., & Masood, M., (1979). LVI. Of Chem. Abatr., Journal of Indian Chemical society. 92: 177470d
22. Adinarayana, D.,(1980). Chem. Abstr., 94:17707lu. Leather science 27(8): 268-270
23. Chopra,R.N.; Chopra,I.C.; Handa K.L.,1994, Indegenous Drug of India. Academic Publication, Calcutta 2nd edition, 2-15,72
24. Dr.Pulok K.Mukhherjee, 2002. Quality Control of Herbal Drugs. An approach to evaluation of botanical 1st edition, Business Horizons New Delhi 2
25. Baxi AJ, Shukla VJ, Bhatt VB. Methods of Qualitative testing of Phytoconstituent, Gujrat Ayurved University Jamnagar 2001:P. 03-15.
26. Prado, W.A., Tonussi, C.R., Rego, E.M., & Corrado, A.P., (1990). Anticonciception induce by intraperitonial injection of gentamicine in rats & mice. Pain. 41: 365-371
27. Mossa, J.S., Rafatullah, S.,Galal,A.M..,Al-Yahya, M.A., (1995) Pharmacological studies of Rhus retinorrharal. International Journal of Pharmacognosy. 33: 242-246
28. NIH consensus conference: Diagnosis & treatment of depression in late life.(1992). Journal of American Medical Association 268(8): 1018-1024.
29. Kar. A., Medicinal Chemistry, edition 3rd , 2005, reprint,2006.194-196
30. Chermat, R., Thierry, B., Mico, J.A., Steru, L.,& Simon, P.,(1986). Adaptation of the tail suspension test to the rat. Journal of Pharmacology. 17(3): 348-350.
31. Cormier S.A. Lomnicki S., Backes W., Dellinger B., et al. 2006. Enviorn. Health Pers. 114(6), 810
32. Goodman & Gilman,(1996). The Pharmacological Basis of Therapeutics, 9th edition McGraw-HILL, Profession Division, Nw York, 959-975.
33. Ramirez, N.N., Ruiz, J.D.Q., Arellano, B.R., Madrigal, M.T.V.& Michel, G.P., (1998) Anticonvulsant effect of Magnolia grandifora L. in the rats. Ethnopharmacology 61: 143-152.
34. Ramnathan, M., Khanna, V.K., Seth, P.K., Jaiswal, A.K., & Bhattacharya, S.K.,(1999).Central neurotransmitter receptor binding & behavior during streptozocin induced diabetes mellitus in rats. Biogenic amines 15(30): 335-365.
35. Kulkarni, S.K., & Joseph, P., (1998). Psychopharmacologal profile of siotone granules, an herbal prepration. Indian Drugs. 35 (9): 536-544.
36. Davies, O.L., Raventos, j., & Walpole, A.L., (1946). A method for evaluation of analgesic activity using rats. British Journal of Pharmacology 1: 225-264.
37. Bhattachrya, S.K., & Raina, M.K., Banerjee, D., & Neogy, N.C.,(1971). Potentiation of morphine & pethidine analgesia by some monoamine oxidase inhibitor. Indian Journal of Experimental Biology 9(2): 257-259.
38. Steru, L., Chermat, R., Thierry, B., & Simon, P., (1985). Tail suspension test: A new method for screening antidepressant in mice. Psychopharmacology 85: 367-370.
39. Turner, R.A., (1965). Analgesic screening method in Pharmacology. In: Turner, R.A., & Hebborn, P., (eds.). Academic press, New York. Pp.100.
40. Hutchinson, J., & alziel, J.M., (1963). In: Hepper, F.N.(ed.). Flora of West Tropical Africa. 2nd edn. London, pp 140-141.
41. Pellow, S., Chopin, P., File, S.E., & Briely, M. (1985). Validation of open : closed arm entries in an elevate plus-maze as a measure of anxiety in rats. Journal of Neurosciences Methods. 35(6): 565-575
42. Goodman & Gilman, (2001). The Pharmacological Basis of Theraputics.10th edn. McGraw- Hill, Medical Publishing division, New York, pp472.
43. Oliver, B., Mos, J., Der Heyden, J.V., Der Poel, G.V., Tulp, M., Slangen, J., & De Jonge, R., (1992). Stress Medicine, 8: 117.
44. Polter, W.Z., Manji, H.K., Osman, O.T., & Rudorfer, M.V.(1992). New prospects in psychiatry: the bioclinical interface. Macher, P.J. & Crocq (eds.) Elsevier, New York, pp. 113.
45. Porsolt, R. D., Bertin, A. & Jalfre, M. (1977a) Behavioral despair in mice: A Primary screening test for antidepressant. Archives International et Pharmacodynamic et de Therapies 229: 327-336.
46. Vogel, G.H., & Vogel, W.H., (1997). Drug discovery & evaluation: Pharmacological assay. Springer Verlag, Berli, pp.292-316, 413.
47. Jonas, W.B. (1997). Research alternative medicine. Natural Medicine. 3(8): 824-827.
48. Dews, P.B. (1953). The measurement of the influence of drugs on voluntary activity in mice. British Journal of Pharmacology. 8: 46-48.
49. Salens, J.K., Kovacsic, G.B. & Allen, M.P. (1968). The influence of adrenergic system on the 24-hrs locomotor activity pattern in mice. Archives International et Pharmacodynamic et de Therapie 173: 411-416.
50. Nakatsu, K. & Owen, J.A. (1980). A microprossesor-basde animal monitoring system. Journal of Pharmacological Methods 3: 71-82.
51. Schumacher, G.A., Goodell, H., Hardy, J. & Wolff, H.G.(1940). Uniformity of pain threshold in man. Science 92:110-112.
52. Goodell, H., Hardy, J. & Wolff, H.G. (1940). Studies on pain. Measurement of the effect of morphine, codeine & other opiates on the pain threshold & an analysis of their relation to the pain experience. Journal of clinical investigation. 19: 659-680.
53. Plummer, J.L., Cmielewski, P.L., Gourlay, G.K., Owen, H. & Cousins, M.J. (1991). Assessment of anticonciceptive drug effects in the presence of impaired motor performance. Journal of Pharmacological Methods. 26:79-87.
54. File, S.E. (1985). Animal Models for predicting clinical efficacy of anxiolytic drugs: social behavior. Neuropsychobilogy 13: 55-62.
55. Stein, M.B. & Uhde, T.W. (1998). Biology of anxiety disorders. In, The American Psychiatric Press Textbook of Psychopharmacology, 2nd edn. (Schatzberg, A.F. & Nemeroff, C.B., eds.) American Psychiatric Press. Washington, D.C., pp. 609-628.
56. International Journal of Pharmaceutical Sciences Review and Research, Review Article PHARMACOLOGICAL POTENTIAL OF PLANT USED AS APHRODISIACS Volume 5, Issue 1, November – December 2010; Article-016
57. Journal of Herbal Medicine & Toxicology, J.Herb.Med.Tox. Vol. 1(1): 1-7 (2007), ST. JOHN’S WORT-NATURE’S BEQUEST, VIKAS KUMAR Department of Pharmaceutics, Institute of Technology, Banaras Hindu University Varanasi 221 005, UP, India.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









