About Authors:
About Authors:
Krishn Kumar Agrawal*, Kishan Singh, Bhupesh Chander Semwal
Institute of Pharmaceutical Research GLA University,
Mathura-281403 (U.P.) India.
*Krishn.agrawal@rediffmail.com
ABSTRACT:
Medicinal plants have been a major source of therapeutic agents since ancient times to cure human disease. Aloes species (family-Liliaceae) is a widely used herbaceous, shrubby, arborescent, perennial or xerophytic succulents medicinal plant cultivated throughout India and popular in various indigenous system of medicine like Ayurveda, Siddha, Unani and Tibb. Traditionally the juice of aloe is used as bitter, laxative, purgative and cathartic. They are also useful in jaundice, dyspepsia, piles, skin disease, abotificient, trophic ulcer, wounds healing and many more ailments. The present review is therefore, an effort to give a detailed survey of the literature on pharmacognosy, phytochemistry and pharmacological activities of the plant.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1403
INTRODUCTION:
Medicinal plants have been a major source of therapeutic agents since ancient times to cure human disease. India is considered as botanical garden of the world and more than 2200 species of medicinal and aromatic plants have been identified after studies. The revival of interest in natural drugs started in last decade mainly because of the wide spread belief that green medicine is healthier than synthetic products. Now-a-days, there is manifold increase in the interest of use of medicinal plants throughout the world which are growing at a rate of 7-15% annually. Despite the major advances in the modern medicine, the development of new drugs from natural products is still considered important.
World Health Organization (WHO) has been encouraging countries to identify and exploit traditional medicine and phyto-pharmaceuticals. As per WHO, about 80% of the population in the world relays on the traditional medicine for the treatment of various disease1. Therefore, the evaluation of rich heritage of traditional medicine is essential. In this regard, one such plant genus is Aloe which is an herbaceous, shrubby, or arborescent, perennial, xerophytic succulents, found in tropical and South Africa, Malagasy and Arabica. The term ‘Aloe’, in medicine is stands for the dried juice which is mainly used in digestive ailments and prepared by cutting the leaves into the suitable vessels and then concentrated by evaporation, either spontaneously or more frequently by boiling.
Aloe, commonly known as Musabbar, is a reputed purgative, cathartic, laxative, and bitter in indigenous system of medicine. Three varieties of aloes are official in the Indian Pharmacopoeia: (1) Curacao aloe obtained from A. Barbadensis(synm. A. vera); (2) Socotrine aloe obtained from A. perryi; and (3) Cape aloe from A. ferox and its hybrids.
Of about 180 known species of aloe, the drug is mainly obtained from the following; cape variety from A. ferox and its hybrid; Curacao variety from A. barbadensis, Socotrine and Zanzibar varieties from A. perryi. The genus Aloe includes herbs, shrubs and trees, bearing spikes of white, yellow or red flower2. The Washington conference of the Convention on International Trade in Endangered Species (CITES) placed all species of aloe, with the exception of A. vera (a cultivator of A. barbadensis), on the protected list3.
PHARMACOGNOSTICAL STUDIES:
Different macroscopic and microscopic characteristic have been found in different species of aloes, which are as follow:
Table 1: MACROSCOPIC CHARACTER4:
|
Characters |
Curacao aloe |
Cape aloe |
Socotrine aloe |
Zanzibar aloe |
|
Odour |
Strong iodoformic |
Sour but distinct |
Unpleasant |
Disagreeable |
|
Taste |
Bitter |
Nauseating and bitter |
Bitter, nauseous |
Bitter |
|
Colour |
Brownish-black, opaque mass |
Dark brown or greenish brown to olive brown |
Brownish yellow |
Liver brown |
|
Appearance |
Waxy, resinous, transparent, uneven fractured surface |
Glassy fractured surface |
Semisolid Opaque mass with conchoidal fractured surface |
Dull, waxy, smooth, and even fractured |
MICROSCOPIC CHARACTER:
Transverse section of aloe leaf show the following characteristic:-
A strong cuticularized epidermis with numerous stomata on both side of surfaces which enclose a region of parenchyma containing chlorophyll, starch and occasional bundles of needle of calcium oxalate. A central region which frequently occupies about three-fifths of the diameter of the leaf, consisting of large, mucilage-containing parenchymatous cells which covers the double raw of vascular bundles which lie at the junction of two previous zones and have a well marked pericycle and endodermis. The aloetic juice from which the aloin is prepared is contained in the large, pericycle cells and sometime in adjacent parenchyma3.
The study showed that the leaf structure, content and the storage location of aloin in the leaves of six species of Aloe were studied by means of semi-thin section, high performance liquid chromatography and fluorescent microscope. Results showed that all leaves cosisted of epidermis, chlorenchyma, aquiferous tissue and vascular bundles with xeromorphic characteristics, including thickened epidermal cell wall, thickened cutical, sunken stomata and well developed aquiferous tissue with the exception of this, there were remarkable differences in leaf structure among the six species. The chlorenchyma cells were similar to palisade tissue in Aloe arborescens and A. Mutabilis, but isodiametric in A. Vera, A. Vera var. Chinensis, A. Saponaria and A. Greenii, A. Arborescens, A. Mutabilis, A. Vera included large parenchymatous cells at the vascular bundles, whereas no such cells were observed at the vascular bundles of A. Saponaria and A. Greenii. In A. Arborescens, A. Mutabilis and A. Vera, the aquiferous tissue sheaths were present and composed of a layer of small parenchymatous cells without chloroplast around the aquiferous tissue. While there were no aquiferous tissue sheaths in A. Vera var. Chinensis, A. Saponaria and A.greenii5.
Table 2: Aloin content and RSD value of different species of Aloe
|
Sr. No. |
Species |
Aloin content (%) |
Relative standard deviation (RSD) |
|
1. |
Aloe arborescens |
0.602 |
1.22 |
|
2. |
A. vera |
0.266 |
1.18 |
|
3. |
A. mutabilis |
0.123 |
1.13 |
|
4. |
A. vera var. Chinensis |
0.011 |
1.26 |
|
5. |
A. saponaria |
0.009 |
1.38 |
|
6. |
A. greenii |
0.076 |
1.24 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
TRADITIONAL USE4,6:
Aloe verais one of the wonderful medicine that used traditionally in various ailments and condition both externally as well as internally which are as follow:
The Aloe vera is an effective natural remedy to cure stomach and esophageal ulcers. Ingestion of Aloe vera is recommended as a home remedy for gastritis, irritable bowel syndrome and ulcerative colitis. Recent studies claim that taking Aloe vera helps control diabetes because it lowers blood glucose levels and remedy for gout and arthritis Aloe vera are recommended by their properties to control uric acid levels. The Aloe vera plant contains substances that increase the defenses helps to generate antibodies against the virus. It is effective natural remedy for cancer sores, gingivitis, periodontitis and oral candidiasis. Aloe vera naturally applied to burns, skin injuries and superficial wounds promotes tissue regeneration. It is effective in psoriasis, eczema, dermatitis, herpes and all types of skin conditions. The Aloe vera in cosmetics is highly valued because it normalizes skin, reduces pores, prevents wrinkles, remove skin blemishes, eliminate acne, you can use the natural gel or products manufactured using the plant as the soap Aloe vera, creams and tonics. Aloe vera naturally applied to the scalp massage as stop drop and significantly improves the hair.
PHYTOCHEMICAL STUDIES:
All the varieties of aloe are the major sources of anthraquinone glycosides. The principle active constituent of aloin is a water-soluble crystalline glycoside barbaloin [10(1)-deoxyglucosyl aloe-emodin anthrone, C21H22O9. H2O, MP 148-1490 (anhyd.)]. Among the other glycoside constituents reported are isobarbaloin and β-barbaloin. Aloes also contain small quantities of free anthraquinones, such as aloe-emodin (hydrolytic product of barbaloin) and isoemodin and resin. The odour is due the presence of traces of essentisl oil.
The raw pulp of A. vera contains approximately 98.5% water, while the mucilage or gel consists of about 99.5% water . The remaining 0.5 – 1% solid material consists of a range of compounds including water-soluble and fat-soluble vitamins, minerals, enzymes, polysaccharides, phenolic compounds and organic acids. Barbados aloe is in considerable demand because of its medicinal and other virtues. It contains small quantities of anthraquinone derivatives like aloe-emodin, 27-30% aloin. The leaves contain barbaloin, chrysophanol glycoside and the aglycone, aloe-emodin. The mucilage of the leaves contains glucose, galactose, mannose and galacturonic acid in addition to an unidentified aldopentose and a protein (0.013%) with 18 amino acids. The plant contains aloesone (7-hydroxychromone, C13H14O4 mp 150-1520) and aloesin (C-glucosyl-7-hydroxychromone)2.The drug also contains aloetic acid, homonataloin, aloesone, chrysophanic acid, chrysamminic acid, galactouronic acid, choline, choline salicylates, saponins, mucopolysaccharides, glucosamines, hexuronic acid, coniferyl alcohol, etc4.
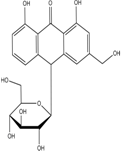
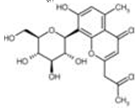
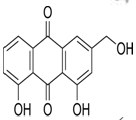
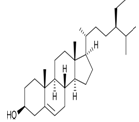
Table 3:- Common chemical constituents present in different species of aloe:
|
Name |
Chemical structure |
Uses |
|
Aloin A |
|
Stimulant laxative, constipation4 |
|
Aloesin |
|
Purgative, radical scavanging action 7 |
|
Aloe emodin |
|
Stimulant laxative, anticancer8 |
|
β- sitosterol |
|
Reduce blood cholesterol level, angiogenic9 |
|
aloeride |
- |
Immunostimulant10 |
|
glycoprotein |
- |
Wound healing11 |
|
Aloe- polymannose |
- |
Anti-coxsackievirus12 |
|
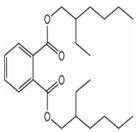
Di(2-ethylhexyl)phthalate |
|
Antileukemic, antimutagenic13 |
|
8-C-beta-D-[2-o (E)-coumaroyl]glucopyranosyl-2-[2-hydroxy]-propyl-7-methoxy-5-methyl chromone |
- |
Antioxidant14 |
PHARMACOLOGICAL STUDIES:
It was proved that A. vera have various therapeutic uses in various ailments such as wound healing, antidiabetic, antiulcer, antiviral, antifungal, antibacterial, antineoplastic and various GI disorder. Various pharmacological studies on A. species are as follows:
Antioxidant activity:
The antioxidant activities of A. vera was determined byHu et al 200315 by determiningthe polysaccharide and flavonoid concentrations of two-, three-, and four-year-old A. vera and compared to BHT and (alpha)-tocopherol by the DPPH radical scavenging method and the linoleic acid system at 100 micrograms of soluble solids per mL of ethanol. The results showed that three-year-old A. vera contained significantly higher levels of polysaccharides and flavonoids than two- and four-year-old A. vera, and no significant differences in flavonoid levels were found between three- and four-year-old A. vera. All the A. extracts showed significant antioxidant activity. The three-year-old extract exhibited the strongest radical scavenging activity of 72.19%, which is significantly higher than that of BHT at 70.52% and (alpha)-tocopherol at 65.20%. These data suggest that the growth stage plays a vital role in the composition and antioxidant activity of A. vera.
Loots et al 200416 identified, quantified, and compared the phytochemical contents and antioxidant capacities of A. ferox lyophilized leaf gel (LGE) and 95% ethanol leaf gel extracts (ELGE) using GC-MS and spectrophotometric methods. Analytically, 95% ethanol is less effective than ethyl acetate/diethyl ether or hexane (in the case of fatty acids) extractions in separating phytochemicals for characterization purposes. Both analytical methods used show the non-flavonoid polyphenols to contribute to the majority of the total polyphenol content. Due to its phytochemical composition, A. ferox leaf gel may show promise in alleviating symptoms associated with/or prevention of cardiovascular diseases, cancer, neurodegeneration, and diabetes
The antioxidant properties in the alcoholic extract of A. vera leaf gel was studied by Rajasekaran et al 200517. Oral administration of A. vera gel extract at a concentration of 300 mg/kg to diabetic rats significantly decreased the levels of blood glucose, glycosylated hemoglobin and increased hemoglobin. The increased levels of lipid peroxidationand hydroperoxides in tissues of diabetic rats were reverted back to near normal levels after the treatment with gel extract. The extract treatment also resulted in a significant increase in reduced glutathione, superoxide dismutase,catalase, glutathione peroxidase and glutathione-S-transferase in the liver and kidney of diabetic rats. These results clearly show the antioxidant property of A. vera gel extract. The extract was also more effective than glibenclamide in restoring the values of these parameters.
The effects of the exudate of A. barbadensis leaves on oxidative stress and some antioxidant status of streptozotocin induced - diabetic rats were studied by Nwanjo 200618. There was significant reduction in scavenging enzymes like superoxide dismutase (SOD) activity and significant increase in signs of oxidative tissue damage, such as lipid peroxidation products (plasma MDA) in streptozotocin induced - diabetic rats. Treatment with A. barbadensis (150mg/kg) increased antioxidant enzymes like SOD activities and significantly reduced lipid peroxidation products. This study shows that high blood sugar leads to increased oxidative stress and that exudates of A. barbadensis leaves possessed antioxidant activity as shown by increased scavenging SOD activity and decreases in lipid peroxidation products levels.
The antioxidant activity of ethanolic extract of A. vera leaf skin was studied by Milandi et al 200819 by fractionated the liquid-liquid partition using hexane, ethyl acetate, chloroform-ethanol and butanol. The total phenolic content of the four different fractions were determined by Folin-Ciocalteu method and their antioxidant activity was assayed through some in vitro models such as the antioxidant capacity by phospho-molybdenum method, β-carotene bleaching method, radical scavenging activity using 2,2-diphenyl-1-picryl hydrazyl (DPPH) assay and reducing power assay. The chloroform-ethanol fraction showed the highest total phenolics (40.500 ± 0.041 μg gallic acid equivalents/g of extract), the highest scavenging activity and the greatest reducing power, followed by ethyl acetate, butanol and hexane extracts.
The antioxidative properties of extracts of A. vera gel made in methanol (MEAG), 95% ethanol (EEAG), hexane (HEAG), acetone (AEAG) and chloroform (CEAG) was studied by Saritha et al 201020 in various in vitro systems viz. 1, 1-diphenyl-2-picrylhydrazyl (DPPH), superoxide anion radicals scavenging, metal ion chelation, reducing power, hydroxyl radicals scavenging and total antioxidant activity in linoleic acid emulsion system. The results showed that MEAG and AEAG possessed maximum DPPH free radical and superoxide radical scavenging activities. All the extracts were effective in scavenging the hydroxyl radicals in nonsite-specific assay as well as in site specific assay. The formation of the Ferrozine- Fe2+ complex was found to be incomplete in the presence of MEAG and AEAG, indicating their capacity in chelating iron. The AEAG was shown more reducing power than MEAG.
The antioxidant properties of A. vera was studied by Khaing 201121 in 95% ethanol and different concentrations of the extracts and ascorbic acid were prepared between 625 and 10000 ppm. 100 μl of each sample was put in the 96-well microtitre plate, added 100 μl of 0.01 mM DPPH solution and incubated for 30 min in the dark. Control was 100μl methanol and 100μl DPPH solution. The quantitative and qualitative determination was made and confirmed that the compounds with antiradical activity changed color as yellow from the purple-blue.
Antidiabetic activity:
The ability of A. vera to lower the blood glucose level was studied by Ghannam et al 198622 in 5 patients with non-insulin-dependent diabetes and in Swiss albino mice made diabetic using alloxan. During the ingestion of A.s, half a teaspoonful daily for 4-14 weeks, the fasting serum glucose level fell in every patient from a mean of 273 +/- 25 (SE) to 151 +/- 23 mg/dl (p<0.05) with no change in body weight. In normal mice, both glibenclamide (10 mg/kg twice daily) and aloes (500 mg/kg twice daily) induced hypoglycaemia after 5 days, 71 +/- 6.2 and 91 +/- 7.6 mg/dl, respectively, versus 130 +/- 7 mg/dl in control animals (p<0.01); only glibenclamide was effective after 3 days. In the diabetic mice, fasting plasma glucose was significantly reduced by glibenclamide and A.s after 3 days. Thereafter only aloes was effective and by day 7 the plasma glucose was 394 +/- 22.0 versus 646 +/- 35.9 mg/dl, in the controls and 726 +/- 30.9 mg/dl in the glibenclamide treated group (p <0.01). We conclude that A.s contains a hypoglycaemic agent which lowers the blood glucose level.
The acute and chronic effects of the exudate of A. barbadensis leaves and its bitter principle was studied by Ajabnoor M A 199023 on plasma glucose levels of alloxan-diabetic mice. Aloes was administered orally, 500 mg/kg, and the bitter principle was administered intra-peritoneally, 5 mg/kg. The hypoglycemic effect of a single oral dose of aloe on serum glucose level was insignificant whereas that of the bitter principle was very highly significant and extended over a period of 24 h with maximum hypoglycemia observed at +8 h. In chronic studies, aloe was administered twice daily and the bitter principle was administered once a day for 4 days. The maximum reduction in plasma glucose level was observed at the 5th day in both cases. The hypoglycemic effect of aloe and its bitter principle may be mediated through stimulating synthesis and/or release of insulin from the beta-cells of Langerhans.
Bolkent et al 200424 was observed thesignificant degenerative changes in the kidney tissue of untreated neonatal streptozotocin (STZ)-induced type-II diabetic rats. These degenerative changes were diminished in the kidney tissue of diabetic animals given glibenclamide and A. leaf gel and pulp extracts. Kidney lipid peroxidation levels were increased in diabetic rats compared to healthy rats; these levels were higher in rats treated with glibenclamide than in those which received Aloe extracts. Serum urea and creatinine levels were higher in diabetic rats in comparison to healthy rats. The administration of aloe gel extract and glibenclamide decreased serum urea and creatinine levels in comparison to diabetic controls. Only A. vera leaf gel extract showed improvement both in histological and biochemical parameters suggesting a protective effect of A. vera on mild damage caused by type-II diabetes on kidney tissue.
The hypoglycemic activity in the alcoholic extract of A. vera gel was studied by Rajasekaran et al 200425 and Effects of oral administration of A. vera extract at a concentration of 200 and 300 mg/kg of body weight on (a) normal fasted rats, (b) oral glucose-loaded rats, and (c) streptozotocin-induced diabetic rats have been studied. A. vera extract maintain the glucose homeostasis by controlling the carbohydrate metabolizing enzymes.
The antidiabetic activity of A.vera in streptozotocin-induced diabetic rats was studied byNoor et al200826. The diabetic induced rats fed with A. vera (300 mg/kg body wt), the fasting plasma glucose levelswere reduced to normal and body weight was found tobe increased. In the pancreatic sections of diabetic ratsfed with A. vera, the islets were comparable to normalrats.
The effects of processed A. vera gel (PAG)on the course of established diet-induced non-insulin-dependent diabetes mellitus (NIDDM) was studied by Kim et al 200927 in C57BL/6Jmice. NIDDM was induced in C57BL/6Jmice by feeding them a high-fat diet. Mice exhibiting diet-induced obesity (DIO) with blood glucose levels above 180mg/dl were selected to examine the anti-diabetic effects of PAG. Oral administration of PAG for 8 weeks reduced circulating blood glucose concentrations to a normal level in these DIO mice. In addition, the administration of PAG significantly decreased plasma insulin. The anti-diabetic effects of PAG were also confirmed by intra-peritoneal glucose tolerance testing. PAG appeared to lower blood glucose levels by decreasing insulin resistance. At the end of the 8-week experimental period, blood samples were taken from the inferior vena cava, and plasma insulin concentration was measured using commercial insulin ELISA kits. Results are means (+/-) SEM (n=18). P<0.01 compared with RD-fed mice. p<0.05, p<0.01 compared with untreated DIO mice. These results demonstrate that the oral administration of PAG prevents the progression of NIDDM-related symptoms in high-fat diet fed mice, and suggest that PAG could be useful for treating NIDDM.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Antimicrobial activity:
The comparative antimicrobial activities of ethanol extract of gel and leaf of A. vera were studied by Agarry et al 200528against Staphylococcus aureus, Pseudomonas aeruginosa, Trichophyton mentagraphytes, T. schoeleinii, Microsporium canis and Candida albicans. Antimicrobial effect was measured by the appearance of zones of inhibition. Antimicrobial susceptibility test showed that both the gel and the leaf inhibited the growth of S. aureus (18.0 and 4.0 mm, respectively). Only the gel inhibited the growth of T. mentagrophytes (20.0 mm), while the leaf possesses inhibitory effects on both P. aeruginosa and C. albicans.
The antimicrobial activities of A. excelsa were studied by Coopoosamy et al 200729. The plant extracts were then tested for antibacterial properties against five strains of gram-positive (Bacillus subtilis, Micrococcus kristinae, Bacillus cereus, Staphylococcus aureus, Staphylococcus epidermidis) andfour strains of gram-negative bacteria (Escherichia coli, Proteus vulgaris, Enterobacter aerogenes, and Shigella Sonnei) forantibacterial activity. The result indicates that leaf extracts of A. excelsa tends to inhibit gram positive bacterial growth for both acetone and ethyl acetate extracts.
The antimicrobial activity of three different extracts of A. vera such as aqueous, ethanol and acetone were studied by Arunkumaret al 200930 by agar diffusion method. The maximum antibacterial activities were observed in acetone extracts (12±0.45nm, 20±0.35nm, 20±0.57nm and 15±0.38nm) other then aqueous extracts and ethanol extract. Antifungal activity of A. vera was analyzed gains Aspergillus flavus and Aspergillus niger. The maximum antifungal activity was observed in acetone extracts (15±0.73nm and 8±0.37nm) when compared other extracts.
In another study the same activity of A. Vera juice with different solvents viz; hexane, ethyl acetate, petroleum ether and ethanol was studied by Thiruppathi et al 201031 against Gram positive bacteria (B. subtilis, S. aureus), Gram negative bacteria (E. coli, K. pneumoniae, P. aeruginosa). The disc diffusion method was used to test the antimicrobial activity.
The antibacterial activity of A. barbadensis was studied by Pandey et al 201032 on clinically isolated bacterial pathogens i.e. Enterococcus bovis, Staphylococcus aureus, Escherichia coli, Proteus vulgaris, Proteus mirabilis, Pseudomonas aeruginosa, Morganella morganii, and Klebsiella pneumoniae causing infection in human being. Ethanolic and aqueous extracts were used for the antibacterial effect, which was measured by the appearance of zone of inhibition. Relatively higher MIC concentrations were obtained for gram negative bacteria E. coli and K. pneumoniae, with ethanol extract; however, no inhibitory effect was noted for aqueous extract. Ethanolic extract possesses great inhibitory activity for gram positive bacteria, E. Bovis followed by S. aureus. Among gram negative bacteria, highest inhibitory effect was observed with P. aeruginosa, followed by M. morganii, P. mirabilis, and P. vulgaris, which was significant (p < 0.01) than E. coli and K. pneumoniae.
The in vitro antibacterial properties of methanolic extract of A. vera gel extract was studied by Saritha et al 201020 and investigated against various common pathogenic bacteria. The gel extract showed significant zone of inhibition against all the pathogens studied and the results were comparable to the conventional antibiotics. Hence it is suggested that A. vera gel extract could be same as a new source of natural antioxidant with potential applications for reducing the levels of lipid oxidation and oxidative stress.
The antimicrobial activity of A. barbadensis leaf was studied by Kohli et al 201133 by using aqueous, ether and solvent extracts. The yellow exudate with aqueous media or acetone and crude sap was used to evaluate the antimicrobial activity. The antimicrobial results showed effective inhibitory action against S. aureus, E. coli, and C. Albicans. The results indicated that crude sap possessed antimicrobial activity.
Cock I E34was studied the fractionated Methanolic extracts of A. barbadensis inner leaf gel by RP-HPLC and the resultant fractions were tested for inhibitory activity against a panel of bacteria and fungi. Five fractions were identified as having antimicrobial activity. Fraction 1 had the broadest antibacterial activity, being capable of inhibiting growth of both Gram-positive and Gram-negative bacteria as well as inhibiting growth of a nystatin resistant strain of the fungus Aspergillus niger. Fraction 1 had similar UV spectral properties as A. emodin and was chromatographically identical to the pure compound. The other fractions tested were much more selective in their antimicrobial activities, being only capable of inhibiting the growth of specific Gram-negative rod bacteria. Two of these antimicrobial fractions were identified by ESI mass spectroscopy as being isomers of 8-C-β-D-[2-0-(E)-coumaroyl] glucopyranosyl - 2-[2-hydroxy]-propyl-7-methoxy-5-methylchromone.
Immunomodulatory activity:
Immunomodulatory properties of A. vera was studied by Madan et al 200835 and result reveled that administration of A. vera extract to swiss albino mice (300 mg/kg i.p.) daily for five days, significantly (P < 0.01) increases the total white blood cells count. Further, it increases humoral immune response, as demonstrated from the increase in plaque-forming cells in the spleen and circulating antibody titre.
The immunomodulatory activity of saline extracts of leaves of A. vera on the albino mice was studied byChandu et al 201136. The saline extract of leaves of A. vera was administered orally according to their body weight in mice. The assessment of immunomodulatory activity on specific and nonspecific immunity was studied by administration of test extract. The method of pyrogallol induced immunosupression was employed with slight modification to study the immunomodulatory potential of the extract. Humoral antibody response to SRBC measurement of antibody titer by haemagglutination reaction was done and cellular immune response (Foot pad reaction test) the edema was induced in the right paw of mice by injecting SRBC (0.025x109 cells) in the sub planar region. Pyrogallol-induced suppression of humoral as well as cell mediated immune response was significantly attenuated by daily oral treatment with saline extract of A. vera. Vitamin E treated group exhibited similar attenuation of the suppression in immune responses. A. vera extract at the dose of 100 mg/kg was found to suppress delayed type hypersensitivity reaction induced by SRBCs in mice. The study demonstrates that A. vera triggers both specific and non-specific responses to a greater extent.
Pugh et al10 was studied a new immunostimulatory polysaccharide called A.ride from commercial A. vera juice. Aloride is between 4 and 7 million Da, and its glycosyl components include glucose (37.2%), galactose (23.9%), mannose (19.5%), and arabinose (10.3%). At 0.5 microg/mL aloride increased NF-kappa B directed luciferase expression in THP-1 human monocytic cells to levels 50% of those achieved by maximal concentrations (10 microg/mL) of LPS. A.ride induced the expression of the mRNAs encoding IL-1beta and TNF-alpha to levels equal to those observed in cells maximally activated by LPS. Acemannan, the major carbohydrate component from aloe, used at 200 microg/mL in the macrophage assay resulted in negligible NF-kappa B activation.
Anticancer activity:
The study of Pecere et al 200037was founded that aloe-emodin (AE), a hydroxyl-anthraquinone present in A. vera leaves, has a specific in vitro and in vivo anti-neuroectodermal tumor activity. The growth of human neuroectodermal tumors is inhibited in mice with severe combined immunodeficiency without any appreciable toxic effects on the animals. The compound does not inhibit the proliferation of normal fibroblasts nor that of hemopoietic progenitor cells. The cytotoxicity mechanism consists of the induction of apoptosis, whereas the selectivity against neuroectodermal tumor cells is founded on a specific energy-dependent pathway of drug incorporation.
The anti-leukimic and anti-mutagenic activities of A. vera was studied by Lee et al 200013. This study examines the anti-tumour effects of di (2-ethylhexyl) phthalate (DEHP) isolated from A. vera, in human and animal cell lines. Its anti-mutagenic effects were examined using Salmonella typhimurium TA98 and TA100 strains. Growth inhibition was specifically exerted by DEHP against three leukaemic cell lines at concentrations below 100 microg mL(-1). At 100 microg mL(-1) DEHP, K562, HL60 and U937 leukaemic cell lines showed growth inhibition of 95, 97 and 95%, respectively. DEHP exhibited an inhibitory activity of 74, 83 and 81%, respectively, in K562, HL60 and U937 cell lines at a concentration of 10 microg mL(-1). At a concentration of 1 microg mL(-1), DEHP exerted an inhibitory activity of 50, 51 and 52%, respectively, in K562, HL60 and U937. In a normal cell line, MDBK, DEHP exerted 30% growth inhibition at a concentration of 100 microg mL(-1), and showed no inhibitory activity at concentrations below 50 microg mL(-1). It was found that DEHP exerted anti-mutagenic activity in the Salmonella mutation assay. The number of mutant colonies of Salmonella typhimurium strain TA98 upon exposure to AF-2 (0.2 microg/plate) decreased in a concentration-dependent manner in the presence of different DEHP concentrations (decreasing to 90.4, 83.9, 75.4, 69.6 and 46.9%, respectively, for DEHP concentrations of 100, 50, 10, 5 and 1 microg/plate). In the case of Salmonella typhimurium strain TA100, DEHP reduced AF-2-induced mutagenicity at 1, 5, 10, 50 and 100 microg/plate to 57.4, 77.5, 80.0, 89.0 and 91.5%, respectively. The isolated compound from A. vera , DEHP, was considered to be the active principle responsible for anti-leukaemic and anti-mutagenic effects in-vitro.
Antifungal activity:
The anti-fungal activity of hydro-alcoholic plant extract was studied by Casian et al 200738 against the mycelial growth of Botrytis gladiolorum, Fusarium oxysporum, Heterosporium pruneti and Penicillium gladioli on Czapek-agar medium. The minimum fungicidal concentration (MFC) varied between 80 and 100 μl/ml, depending on the fungal species.
The antifungal activity of A. excelsa leaf latex was studied by Coopoosamy et al 200729. The plant materials were soaked in ethanol (95%) and in distilled water in large conical flask for 3 weeks. The extracts (aqueous and ethanol) obtained were evaporated at reduced pressure (450C) to a syrupy residue. The solutions were then tested for antifungal activity using the following fungal cultures: Aspergillus flavus, Aspergillus glaucus, Candida albicans, Candida tropicalis, Trichophyton mentagrophytes, and Trichophyton rubrum. The antifungal activity of the ethanol extracts of A. excelsa was found to be quite impressive as compared to aqueous extracts.
The Antifungal Activity of A. vera leaf extract was studied by Khaing 201121 by agar well diffusion method. The fungal strains were cultured in their respective broths in a water-bath shaker for 48 hr below 30 °C. After incubation, they were harvested and 100 μl of fugal suspensions were swabbed onto SDA (Sabouraud’s Dextrose Agar) medium. Agar wells were carried out by punching out equally spaced wells on the medium that has been heavily seeded with the test organisms. Then, the crude leaf extracts were dissolved in methanol, ethanol and ethyl acetate and filled in the wells and 70 % ethanol was used as control. After filling, the plates were inoculated at 37 °C for overnight and inhibition zones (in mm) were determined.The methanol and ethanol portions of aloeleaf extract were shown to display antifungal activity against all tested fungi except Candida albicans ranging between 11 and 18 mm, while ethyl acetate portion demonstrated antifungal activity exhibited only against three fungi Penicillium maneffei, Fusarium oxysporum and Phythium sp. at the tested concentration. Among three portions of A. vera extract, methanol portions revealed highest antifungal activity.
Hepatoprotective activity:
The hepatoprotective activity of A. barbadensis was studied by Chandan et al 200739. The shade dried aerial parts of A. barbadensis were extracted with petroleum ether (AB-1), chloroform (AB-2) and methanol (AB-3). The plant marc was extracted with distilled water (AB-4). AB-1 and AB-2 were observed to be devoid of any hepatoprotective activity. Out of two active extracts (AB-3 and AB-4), the most active AB-4 was studied in detail. AB-4 showed significant hepatoprotective activity against CCl4 -induced hepatotoxicity as evident by restoration of serum transaminases, alkaline phosphatase, bilirubin and triglycerides. Hepatoprotective potential was confirmed by the restoration of lipid peroxidation, glutathione, glucose-6-phosphatase and microsomal aniline hydroxylase and amidopyrine N-demethylase towards near normal. Histopathology of the liver tissue further supports the biochemical findings confirming the hepatoprotective potential of AB-4. The study showed that the aqueous extract of A. barbadensis was significantly capable of restoring integrity of hepatocytes indicated by improvement in physiological parameters, excretory capacity (BSP retention) of hepatocytes and also by stimulation of bile flow secretion. AB-4 did not show any sign of toxicity up to oral dose of 2 g/kg in mice.
The Hepatoprotective activity was studied by Nayak et al 201140 against Paracetamol- induced elevated level of AST , ALT and ALP and depleted the liver thiol levels when compared with vehicle treated group. Single day treatment with the aqueous extract of A. vera in the dose of 250 and 500mg/kg reduced the AST, and ALT levels significantly (p<0.01). 500mg /kg of the extract also reduced the ALP levels and restored the depleted liver thiol levels significantly (p<0.01), however there was no effect on alkaline phosphatase and liver thiols by A. vera in the dose of 250mg/kg.Seven day treatment with both the doses of A. vera significantly reduced the levels of AST, ALT and ALP significantly (p<0.01) and restored the depleted liver thiol levels significantly (p<0.01).
Anti-inflammatory activity:
The anti-inflammatory activity of A. vera and gibberellin was measured by Davis et al 198941 in streptozotocin-induced diabetic mice by measuring the inhibition of polymorphonuclear leukocyte infiltration into a site of gelatin-induced inflammation over a dose range of 2 to 100 mg/kg. Both aloe and gibberellin similarly inhibited inflammation in a dose-response manner. The result was suggested that gibberellin or a gibberellin-like substance is an active anti-inflammatory component in A. vera.
Vazquez et al 199642 was studied the effects of aqueous, chloroform, and ethanol extracts of A. vera gel on carrageenan-induced edema in the rat paw, and neutrophil migration into the peritoneal cavity stimulated by carrageenan. The aqueous and chloroform extracts decreased the edema induced in the hind-paw and the number of neutrophils migrating into the peritoneal cavity, whereas the ethanol extract only decreased the number of neutrophils. The anti-inflammatory agents indomethacin and dexamethasone also decreased carrageenan-induced edema and neutrophil migration. The aqueous extract inhibited prostaglandin E2 production from [14C] arachidonic acid. These results demonstrated that the extracts of A. vera gel have antiinflammatory activity and suggested its inhibitory action on the arachidonic acid pathway via cyclooxygenase.
Hutter et al 199643 was identified a new anti-infllammatory agentas 8-[C-beta-D-[2-O-(E)-cinnamoyl]glucopyranosyl]-2- [(R)-2-hydroxypropyl]-7-methoxy-5-methylchromone (1) has been isolated from A. barbadensis. At a dose of 200 microg/mouse ear, 1 exhibited topical antiinflammatory activity equivalent to 200 microg/ear of hydrocortisone. There was no reduction in thymus weight caused by treatment with 1 for any of the doses tested, while 200 microg/ear of hydrocortisone resulted in a 50% decrease in thymus weight.
Langmead et al 200444 studied the anti-inflammatory activity of A. vera gel and was founded the dose-dependent inhibitory effect on reactive oxygen metabolite production; 50% inhibition occurred at 1 in 1000 dilution in the phycoerythrin assay and at 1 in 10-50 dilution with biopsies. A. vera inhibited the production of prostaglandin E2 by 30% at 1 in 50 dilution (P = 0.03), but had no effect on thromboxane B2 production. The release of interleukin-8 by CaCO2 cells fell by 20% (P < 0.05) with A. vera diluted at 1 in 100, but not at 1 in 10 or 1 in 1000 dilutions.
The anti-inflammatory activities of A. buettneri was studied by Metowogo et al 200845 by injection of 0.1ml of formaldehyde1% induced rat paw oedema. The hydro-alcohol extract of A. buettneri administrated orally at 100, 250, 500 mg/kg 30 minutes before the formaldehyde injection inhibited oedema 3.14%; 28.26% and 46.22% (p < 0.05), respectively, one hour after oedema induction. Three hours later, inhibition was 82.52; 86.87; 89.81% (p < 0.01), respectively .For the curative studies, the increase in oedema paw volume was 46.72 ±1.34% in thecontrol rats, but was only 26.92 ± 0.66; 15.22 ±2.48; 12.77±1.29% three hours after induction in the rats which received 100, 250, 500 mg/kg of A. extract.
The anti-inflammatory activities of A. barbadensis whole leaf using aqueous, ether and solvent extracts was studied by Kholi et al 201133. The anti-inflammatory activity was studied in both gel & sap of the leaf by formalin induced paw edema method .Diclofenac sodium ointment was taken as the standard drug. The percentage inhibition of paw edema was observed to be 80-95% (Gel), 49.04 (sap) compared to 88.02% as in case of Diclofenac sodium ointment.The results indicated that gel of A. leaf possessed significant anti-inflammatory activity.
Wound healing activity:
The wound healing activity of A. vera was studied by Davis et al 198946. The Wounds were induced on both sides of the vertebral column of ICR mice using a biopsy punch. For the oral study, experimental animals received A. vera in their drinking water for two months, whereas the control animals received only water. In the topical study, experimental animals were given 25% A. vera in Eucerin® cream topically. The control animals received cream only. A 62.5% reduction in wound diameter was noted in mice receiving 100 mg/kg/day oral A. vera and a 50.80% reduction was recorded in animals receiving topical 25% A.vera.
The wound healing activity of A. vera in diabetic rats was studied byChithra et al 199847. Full-thickness excision/incision wounds were created on the back of rats, and treated either by topical application on the wound surface or by oral administration of the A. vera gel to the rat. Wound granulation tissues were removed on various days and the collagen, hexosamine, total protein and DNA contents were determined, in addition to the rates of wound contraction and period of epithelialization. Measurements of tensile strength were made on treated/untreated incision wounds. The results indicated that A. vera treatment of wounds in diabetic rats may enhance the process of wound healing by influencing phases such as inflammation, fibroplasia, collagen synthesis and maturation, and wound contraction.
The wound healing activity of glycoprotein fraction from A. vera was studied by Choi et al 200111 and named G1G1M1DI2. It showed a single band on sodium dodecyl sulphate-polyacrylamide gel electrophoresis, with an apparent molecular weight of about 5.5 kDa. It exhibited significant [3H] thymidine uptake in squamous cell carcinoma cells. The effect of G1G1M1DI2 on cell migration was confirmed by accelerated wound healing on a monolayer of human keratinocytes. When this fraction was tested on a raft culture, it stimulated the formation of epidermal tissue. Furthermore, proliferation markers (epidermal growth factor receptor, fibronectin receptor, fibronectin, keratin 5/14 and keratin 1/10) were markedly expressed at the immunohistochemical level. The glycoprotein fraction enhanced wound healing in hairless mice by day 8 after injury, with significant cell proliferation. The result showed that this glycoprotein fraction is involved in the wound-healing effect of A. vera via cell proliferation and migration.
The angiogenic activity of beta sitosterol from A. vera was studied by Choi et al 20029 on damaged blood vessels ofthe Mongolian gerbil. In a chick embryo chorioallantoic membrane assay, beta-sitosterol was found to have an angiogenic effect. It enhanced new vessel formation in gerbil brains damaged by ischaemia/reperfusion, especially in the cingulated cortex and septal regions, in a dose-dependent fashion (up to 500 microg/kg, p less than 0.05, n = 34 - 40). beta-Sitosterol also enhanced the expressions of proteins related to angiogenesis, namely von Willebrand factors, vascular endothelial growth factor (VEGF), VEGF receptor Flk-1, and blood vessel matrix laminin (p less than 0.05, n = 6). In addition, the intraperitoneal administration of beta-sitosterol at 500 microg/kg/day for a period of 19 days significantly improved the motion recovery of ischaemia/reperfusion-damaged gerbils as assessed by rota-rod testing (p less than 0.001, n = 10). Results suggest that beta-sitosterol has therapeutic angiogenic effects on damaged blood vessels.
The wound healing activity of A. ferox and A. arborescens was studied byJia et al 200848.In rat model, wound severity scores of locally treated group-I and -II proved to be significantly lower than that in the control group on Day-1 and -3 (p < 0.05). It was found that all rats, except one in group-III, recovered from anesthesia or surgery, or both, 18–24 h after the injury. On Day-1 after injury, the incision in group-I was well approximated to each other but not completely epithelialized, meanwhile, showed no signs of inflammation. The healing of group-II, characterized by a small amount of yellow exudates and a discernable unjoined seam on the edge of the wound, was not yet complete. In group-III, a poor wound condition was observed and it was featured by bleeding and skin swelling.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Analgesic activity:
The analgesic activity of A. buettneri was studied by Metowogo et al 200845. Analgesia was evaluated by immersing the tail of the rats in maintained hot water at 50°C. The time of immersion of the tail increased with the dose. It was 4.15 ± 0.17 seconds for control rats, and 5.16 ± 0.21; 7.04± 0.25; 7.07± 0.28 seconds, respectively, for the rats which received 100, 250 and 500 mg/kg of the extract. Thus the doses of 250 and 500mg/kg had practically the same effect.
Antipyretic activity:
The antipyretic activity of A. buettneri was studied by Metowogo et al 200845 by injection of 15% of Brewers' yeast at 10 ml/kg increased the rectal temperature of the rats for 1.5°C. This hyperthermia remained the same for control rats with small fluctuations over 24–hour period. Extract was administered, 18 hours after the induction of hyperthermia, decrease the temperature from 38.78 ± 0.024°C to 38° ± 0.05°C three hours after the administration of the extract. The reduction was dose- and time-dependent in that 6 hours after the administration of the extract, the recorded temperature was 38.70 ±0.3°C for the control while it was 37.70 ± 0.07°C; 37.00 ±0.03°C and 36.85 ± 0.15°C, for rats which received the extract at doses of 100; 250 and 500 mg/kg, respectively
Antiulcer activity:
The effect of A. vera on gastric acid secretion and acute gastric mucosal injury in rats was studied by Yusuf et al 200449 by 0.6 M HCl, pylorus ligated and lumen perfuse rats. Acid secretion was determined by titration of the collected gastric juice to pH 7.0. Intraperitoneal injection of A. vera, dose dependently inhibited gastric acid secretion. The plant was more active as a gastroprotective agent at lower concentration against mucosal injury induced by 0.6 M HCl. In conclusion, A. vera is endowed with gastric acid anti-secretory activity and could protect the gastric mucosa at low concentrations against injurious agents.
The anti-ulcerogenic potential of the leaf methanol extract of A. buettneri was investigated by Tan et al 200650 by using three methods of gastric lesion induction in experimental Wistar rats (150-200 g) and mice (20-25 g): 1. HCl/ethanol-induced gastic lesions, 2. Indomethacin-HCl/ethanol-induced gastric lesions, and 3, Pylorus ligation-induced gastric lesions. The dose-dependent reduction of lesion formation was accompanied by a significant increase in gastric mucus production in mice. Inhibition of lesion formation was 22 and 54 % in mice, 25 and 77% in rats for the doses of 500 and 1000 mg/kg when the HCl/ethanol mixture was given. Pre-treatment, by oral route, with indomethacin significantly reduced the ability of the extract to inhibit the formation of HCl/ethanol-induced lesions, inhibition dropping to 11% for the dose of 1000 mg/kg. When the rats were subjected to pylorus ligation, the level of lesion inhibition was 36 and 68% for the two doses of extract. Gastric acid secretion reduced to 88 and 79mEq/l compared with105 mEq/l for the controls. These results confirm the ethnomedical use of A. buettneri in the management of gastroduodenal ulcer disease.
The antiulcer activity of A. buettneri was studied by Metowogo et al 200845 by the administration of water-HCl-ethanol mixture produced ulceration of 19±1.00 units 1 hour after induction, ethanol only induced ulceration of 9.20±0.58 units 2 hours after induction. A. buettneri extract inhibited dose-dependently the gastric lesion with the percentage of inhibition 25.16 ±11.21; 63.77 ±11.67 and 74.48 ±15.23%, respectively, for rats which received 500, 1000 and 2000 mg/kg of extract. The doses of 1000 and 2000 mg/kg had a significant effect on the gastric lesions (p <0.01). For ulcerations induced by oral administration of ethanol 95%, the length of ulceration decreased from 9.20 ± 0.58 units (day 0) to 5.40 ± 0.51 units (day 4) and to 0.00 unit (day 8) for control animals. These dimensions decreased to 7.20 ±1.20; 1.00 ± 0.45 and 0.00 ± 0.00 units, respectively, on day 2, day 4 and day 6 in the rats treated with the hydro-alcohol extract of A. buettneri. These results show that the extract accelerated the cicatrization of the ulcerous wounds
Antihypertension activity:
Hypotensive Effect of A. barbadensiswas studied by Saleem et al 200451.Hypotensive effects of aloe-emodin, aloin A, elgonica dimer A and bisbenzopyran from A. barbadensis have been studied. Aloe-emodin has emerged as a potent hypotensive agent in current pharmacological investigations and caused 26 %, 52 %, and 79 % falls in mean arterial blood pressure at the corresponding doses of 0.5, 1, and 3 mg/kg in rats.
Antileishmanial activity:
The invitro anti-leishmanial activity of A. vera leaf (AVL) exudate was studied by Dutta et al 200352. Irrespective of the disease manifestation, promastigotes from strains responsible for cutaneous, mucocutaneous, and visceral leishmaniasis were susceptible to AVL and their IC50 ranged from 100-180 μg/ml. In axenic amastigotes cultured from a L. donovani strain 2001 responsible for visceral leishmaniasis, the IC50 was 6.0 μg/ml. AVL caused activation of host macrophages evident by an increased release of members of reactive oxygen species that was attenuated by pre incubation with free radical scavengers. Collectively the data indicates that AVL, via its direct leishmanicidal activity which can be further enhanced by activation of host macrophages, is an effective anti-leishmanial agent.
Clinical study on Aloe vera:
Langmead et al 200453
performed a double-blind, randomized, placebo-controlled trial of the efficacy and safety of A. vera gel for the treatment of mildly to moderately active ulcerative colitis. Clinical remission, improvement and response occurred in nine (30%), 11 (37%) and 14 (47%), respectively, of 30 patients given A. vera, compared with one (7%) [P = 0.09; odds rati o, 5.6 (0.6-49)], one (7%) [P = 0.06; odds ratio, 7.5 (0.9-66)] and two (14%) [P < 0.05; odds ratio, 5.3 (1.0-27)], respectively, of 14 patients taking placebo. The Simple Clinical Colitis Activity Index and histological scores decreased significantly during treatment with A. vera (P = 0.01 and P = 0.03, respectively), but not with placebo. Sigmoidoscopic scores and laboratory variables showed no significant differences between A. vera and placebo. Adverse events were minor and similar in both groups of patients.
Su et al 200454
performed Phase II double-blind randomized study comparing oral A. vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms.By the end of treatment, the two groups were also statistically identical in maximal grade of toxicity, duration of Grade 2 or worse mucositis, quality-of-life scores, percentage of weight loss, use of pain medications, hydration requirement, oral infections, and prolonged radiation breaks.
U.S. Regulatory approval:
Aloe was approved in 1975 by the FDA as an over-the-counter drug to treat constipation proposed as Category 3 in 1998. The FDA required manufacturers to submit additional safety data by June 21, 1999, and it may restrict over-the-counter sale of aloe latex for constipation55.
CONCLUSION:
From the ancient time the plant were the major sources of any disease treatment. In recent years, ethno pharmaceuticals and traditional uses of natural compounds, especially of plant origin received much attention as they are well tested for their efficacy and generally believed to be safe for human use because of less or no side effects. It is a best classical approach in the search of new lead molecules for diagnosis and treatment of various diseases. Thorough screening of literature available on Aloe depicted the fact that it is a popular remedy among the various ethnic groups, vaidhyas, hakims and Ayurvedic, allopathic practitioners for cure of ailments. It is needed to explore other therapeutic potential of this plant very thoroughly.
REFERENCES:
1. Padmaa MP: Nigella sativa Linn. A comprehensive review. Indian J natural products and resourses 2010; 1: 409-429
2. Aloe vera In. Sastri BN (chief editor) The Wealth of India.(vol.5) New Delhi National Institute of Science Communication CSIR, 191-193
3. Evans WC. Trease and Evans pharmacognosy, saunders- an imprint of Elsevier limited, 15th edition 2001, 493, 240-243.
4. Kokate CK, Purohit AP, and Gokhale SB. General Introduction In. Pharmacognosy Pune Nirali Prakashan, 2005, 181-186
5. LI jing yuan, WANG tai-xia, SHEN zong-Gen and HU Zheng-Hai: Relationship between leaf structure and aloin content in six specoes of aloe L. Acta Botanica Sinica 2003; 45: 594-600.
6. Asolkar LV, Kakkar KK, Chakre OJ. Glossary of Indian medicinal plants with active principle part 1New Delhi National Institute of Science communication & Information Resource CSIR 2005,
7. Yagi A, Kabash A, Okamura N, Haraguchi H, Moustafa SM and Khalifa TI: Antioxidant, free radical scavenging and anti-inflammatory effects of aloesin derivatives in Aloe vera. Planta-Med 2002; 68: 957-960.
8. Wasserman L, Avigad S, Beery E, Nordenberg J and Fenig E: The effect of aloe emodin on the proliferation of a new merkel carcinoma cell line. American J Dermatopathol. 2002; 24: 17-22.
9. Choi S, Kim, KW, Choi JS, Han ST, Park YI, Lee SK et al : Angiogenic activity of beta-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta-Med. 2002; 68: 330-335.
10. Pugh N, Ross SA, ElSohly MA and Pasco DS: Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J Agriculture Food Chem. 2001; 49:1030-1034.
11. Choi SW, Son BW, Son YS, Park YI, Lee SK and Chung MH: The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. British J Dermatol 2001; 145:535-545.
12. Gauntt CJ, Wood HJ, McDaniel HR and McAnalley BH: Aloe polymannose enhances anti-coxsackievirus antibody titres in mice. Phytother Res 2000; 14: 261-266.
13. Lee KH, Kim JH, Lim DS and Kim CH: Anti-leukaemic and anti-mutagenic effects of di(2-ethylhexyl)phthalate isolated from Aloe vera Linn. J Pharm Pharmacol. 2000; 52: 593-598.
14. Lee KY, Weintraub ST and Yu BP: Isolation and identification of a phenolic antioxidant from Aloe barbadensis. Free Radic Biol Med. 2000; 28: 261-265.
15. Hu Y, Xu J and Hu Q: Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis Miller) extracts. J agricultural food chemistry. 2003; 51:7788-7791.
16. Loots DT, Westhuizen van der FH and Botes L: Aloe ferox Leaf Gel Phytochemical Content, Antioxidant Capacity, and Possible Health Benefits. J Agriculture Food Chemistry 2007; 55:6891–6896.
17. Rajasekaran S, Sivagnanam K and Subramanian S: Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 2005; 57:90-96.
18. Harrison UN: Antioxidant activity of the exudate from Aloe barbadensis leaves in diabetic rats, Biokemistri 2006; 18:77-81.
19. Miladi S and Damak M: In-vitro antioxidant activities of Aloe vera leaf skin extracts. Journal de la Societe Chimique de Tunisie 2008; 10:101-109.
20. Saritha V, Anilakumar KR and Farhath Khanum: Antioxidant and antibacterial activity of Aloe vera gel extracts. International J Pharmaceutical Biological Archives 2010; 1:376-384.
21. Khaing TA: Evaluation of the Antifungal and Antioxidant Activities of the Leaf Extract of Aloe vera (Aloe barbadensis Miller), World Academy Science, Engineering and Technology 2011; 75:610-612.
22. Ghannam N, Kingston M, Al-Meshaal IA, Tariq M and Parman NS: Antidiabetic Activity Of Aloes: Preliminary Clinical & Experimental Observations. Woodhouse N Horm Res 1986; 24:288-294.
23. Ajabnoor MA, Effect of Aloes On Blood Glucose Levels In Normal & Alloxan Diabetic Mice, J Ethnopharmacol 1990; 28:215-220.
24. Bolkent S, Akev N, Ozsoy N, Sengezer-Inceli M, Can A, Alper O et al: Effect of Aloe vera (L.) Burm. fil. leaf gel and pulp extracts on kidney in type-II diabetic rat models. Indian J Exp Biol. 2004; 42: 48-52.
25. Rajasekaran S, Sivagnanam K, Ravi K and Subramanian S: Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food. 2004; 7: 61-66.
26. Noor A, Gunasekaran S, Manickam AS and Vijayalakshmi MA: Antidiabetic activity of Aloe vera and histology of organs in streptozotocin induced diabetic rats, Current science 2008; 94:1070-1076.
27. Kwanghee K, Hyunyul K, Jeunghak K, Sungwon L, Hyunseok K, Sun-A I, et al: Hypoglycemic and hypolipidemic effects of processed Aloe vera gel ina mouse model of non-insulin-dependent diabetes mellitus, Phytomedicine 2009; 16:856–863.
28. Agarry OO, Olaleye MT and Bello-Michael CO: Comparative antimicrobial activities of Aloe vera gel and leaf. African J Biotechnology 2005; 4:1413-1414.
29. Coopoosamy RM and Magwa ML: Traditional use, antibacterial activity and antifungal activity of crude extract of Aloe excelsa, African J Biotechnology 2007; 6:2406-2410.
30. Arunkumar S and Muthuselvam M: Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens, World Agricultural Sciences 2009; 5:572-576.
31. Thiruppathi S, Ramasubramanian V, Sivakumar T and Thirumalai AV: Antimicrobial activity of Aloe vera (L.) Burm. f. against pathogenic Microorganisms. J Biosci Res 2010; 1:251-258.
32. Pandey R and Mishra A: Antibacterial Activities of Crude Extract of Aloe barbadensis to Clinically Isolated Bacterial Pathogens. Applied Biochemistry and Biotechnology. 2010; 160:1356-1361.
33. Kohli S, Kumari C and Verma SK: Phyto-Chemical Investigation and Therapeutic Evaluation Of Aloe Barbadensis, International J Drug Discovery and Herbal Res Earch 2011; 1:32-34.
34. Cock IE: Antimicrobial Activity of Aloe barbadensis Miller Leaf Gel Components, The Internet J Microbiology.
35. Madan J, Sharma AK, Inamdar N, Rao HS and Singh R: Immunomodulatory properties of aloe vera gel in mice, International J green pharmacy. 2008; 2:152-154.
36. Chandua AN, Kumar CS, Bhattacharjee C, debnath S and Kannan KK: Studies on immunomodulatory activity of Aloe vera (Linn). International J applied biology and pharmaceutical technology 2011; 2:19-22.
37. Teresa Pecere M, Vittoria G, Carla M, Cristina P, Francesca DV, Andrea C et al: Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal Tumors. Cancer Research 2000; 60:2800–2804.
38. Oana RC, Marcel P, Laurian V and Mircea T: Antifungal activity of Aloe vera leaves, Fitoterapia 2007; 78:219–222.
39. Chandan BK, Saxena AK, Shukla S, Sharma N, Gupta DK, Suri KA et al: Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacology 2007; 111:560-566.
40. Nayak V, Gincy TB, Prakash M, Joshi C, Rao SS, Somayaji SN, Madhav NV, Bairy KL: Hepatoprotective activity of Aloe vera Gel against Paracetamol Induced Hepatotoxicity in albino rats. Asian J Pharm Biol Res 2011; 1:94-98
41. Davis RH and Maro NP: Aloe Vera and Gibberellin anti-inflammatory activity in diabetes. J American Podiatric Medical Association 1989; 79:1-4.
42. Vazquez B, Avila G, Segura D and Escalante B: Anti-inflammatory activity of extracts from Aloe vera gel. J Ethnopharmacol. 1996; 55: 69-75.
43. Hutter JA, Salman M, Stavinoha WB, Satsangi N, Williams RF, Streeper RT and Weintraub ST: Anti-inflammatory C-glucosyl chromone from Aloe barbadensis, J Nat Prod. 1996; 59:541-543.
44. Langmead L, Makins RJ and Rampton DS: Anti-inflammatory effects of Aloe vera gel in human colorectal mucosa In-vitro. Aliment Pharmacol Ther. 2004 ;19: 521-527.
45. Metowogo K, Agbonon A, Eklu-Gadegbeku K, Aklikokou AK and Gbeassor M: Anti-ulcer and Anti-inflammatory Effects of Hydroalcohol Extract of Aloe buettneri A. Berger (Lilliaceae), Tropical J Pharmaceutical Research 2008; 7: 907-912.
46. Davis RH, Leitner MG, Russo JM and Byrne ME: Wound healing. Oral and topical activity of Aloe vera. J Am Podiatr Med Assoc. 1989; 79:559-62.
47. Chithra P, Sajithlal GB and Chandrakasan G: Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998; 59: 195-201.
48. Jia Y, Zhao G and Jia J: Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing, J Ethnopharmacology 2008; 120: 181-189.
49. Yusuf S, Agunu A and Diana M: The effect of Aloe vera A. Berger (Liliaceae) on gastric acid secretion and acute gastric mucosal injury in rats. J Ethnopharmacol. 2004; 93: 33-37
50. Tan PV, Enow-Orock GE, Dimo T, Nyasse B and Kimbu SF: Evaluation of the anti-ulcer and toxicity profile of aloe buettneri in laboratory animals. African J Traditional, Complementary and Alternative Medicines 2006; 3: 8-20.
51. Saleem R, Faizi S, Shaheen Siddiqui B, Ahmed M, Hussain SA, Qazi A et al: Hypotensive Effect of Chemical Constituents from Aloe barbadensis, Planta Med. 2001; 67: 757-760.
52. Dutta A, Mandal G, Mandal C and Chatterjee M, In-vitro antileishmanial activity of Aloe vera leaf exudate: A potential herbal therapy in leishmaniasis, Glycoconjugate J 2003; 24:181-86.
53. Langmead L, Feakins RM, Goldthorpe S, Holt H, Tsironi E, De-Silva A et al: Randomized, double-blind, placebo-controlled trial of oral Aloe vera gel for active ulcerative colitis, Aliment Pharmacol Ther. 2004; 19:739-747.
54. Su CK, MehtaV, Ravikumar L, Shah R, Pinto H, Halpern J et al: Phase II double-blind randomized study comparing oral Aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms, Int J Radiat Oncol Biol Phys. 2004; 60:171-177.
55. Sikarwar MS, Patil MB, Sharma S and Bhat V: Aloe vera: Plant of Immortality, International J Pharma Sciences and Research 2010; 1:7-1.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE