About Author
Nrusingha Panda
Department of Quality Assurance,
Mankind Pharma Ltd., Bermiok Elaka, South Sikkim, Sikkim, India
ABSTRACT
Cleaning process has a huge importance in pharmaceutical industries to avoid contamination and cross contamination. To evaluate capacity and effectiveness of a cleaning process, proper techniques should be used for proper sampling, since improper techniques used in sampling may lead to wrong results of previous residues. Sampling also depends upon trained sampler, so training of the sampler is also important. The review focused on swab and rinse sampling techniques used mostly in pharmaceutical industries.
INTRODUCTION
AVAILABLE SAMPLING METHODS
Cleaning refers to a process by which any processing area, article, equipment, part of equipment, accessory etc. are freed of any previous product residue, unwanted particle from the respective surfaces. A cleaning process can be developed with or without utilisation of a detergent. Cleaning process should be designed in such a way that the cleaning process itself does not present a cross-contamination risk. The operational parameters that describe a cleaning process are: Cleaning agent, Concentration, Contact time and Temperature. Based on these parameters, product characteristics and available resources (e.g. for automated cleaning system like Clean-in-place (CIP), Clean-Out of Place (COP) and manual cleaning process), a suitable cleaning process should be designed to clean respective equipment/ area/ item to a certain acceptable level of residue to prevent contamination or cross-contamination. To determine and establish a successful cleaning process, a suitable sampling procedure should be established with a suitable testing procedure. To determine the effectiveness of a cleaning procedure also a suitable sampling procedure should be established. A sampling procedure and a validated testing method should be there to detect the presence of previous residue and residual cleaning agent used in the cleaning process.
Proper sampling methods and analytical tests play an important role in establishing reproducibility and effectiveness of a cleaning procedure.
Various sampling methods are available such as:
a) Swab sampling
b) Rinse Sampling
c) Placebo sampling
d) Coupon sampling
From the above sampling methods, generally swab sampling and rinse sampling methods are used in pharmaceutical industries. Sampling should be carried out by swabbing and/or rinsing or by other means depending on the production equipment. While the FDA guidance indicates a preference for the more direct swabbing method, more recent communication from the International Conference on Harmonisation (ICH) ICH Q7A states that sampling methods need to be comprehensive enough to quantitate both soluble and insoluble residues that are left behind on the surfaces after cleaning. The sampling materials and method should not influence the result.
SWAB SAMPLING
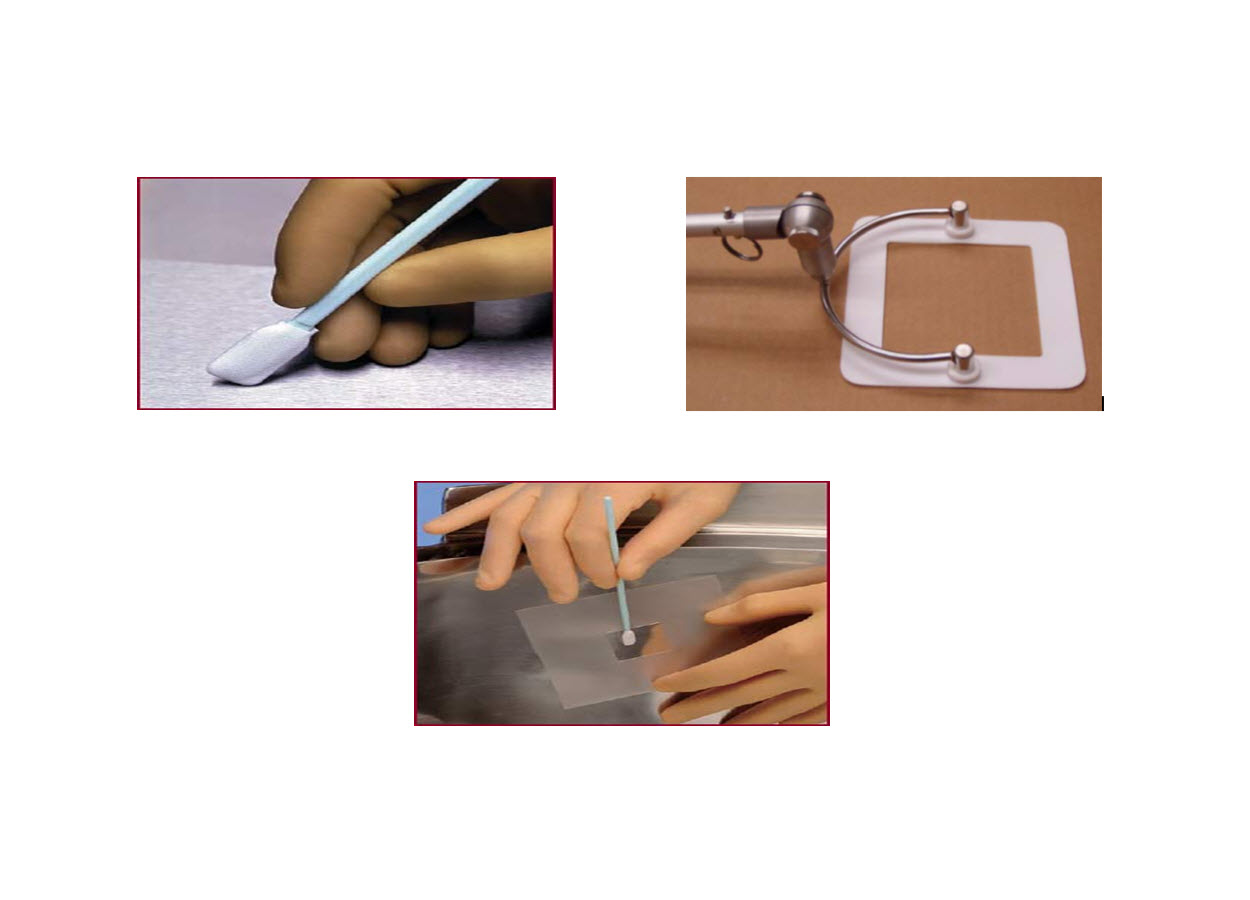
This procedure utilizes fibrous material for swabbing a surface for sampling purpose. The sampling method is also known as direct surface sampling method. The fibrous material of a swab is woven or nonwoven textiles attached to a plastic handle (Figure-1). In this method, a swab (i.e. fibrous material) is first pre wetted with a suitable solvent in which the residue that is to be sampled is soluble. Squeezing the sides of swab head against the sides of vial or test tube upon pre wetting prior to sampling is generally done to remove extra solvent from the swab. This is important since excess solvent may leave extractable substances of interest on the surface which can act as a source of residues leading to variable results. After sampling is done over a predefined area, the handle of the swab is broken and swab head is put into a suitable extraction solvent where the analyte to be analysed and measured is extracted into the solvent. The extracted solvent used may be same or different as that of the solvent used for wetting the swab.
Recovery of swab sample should be considered a major concern, since swab recovery study demonstrates that if a residue is present on an equipment surface can be adequately measured and quantified by the use of analytical method and sampling method. Recovery study is a scientific approach having an objective of establishing reproducible level of recovery from the equipment surface. Recovery should be shown to be possible from all product contact materials sampled in the equipment with all the sampling methods used. Recovery of residues from surfaces depends on the size and shape of the swab head, as well as the properties (such as flexibility and length) of the swab handle. Sampling recovery studies are laboratory studies involving coupons of sampled equipment of different materials of construction (such as stainless steel, glass, PTFE, and EPDM) spiked with residues to be measured. Acceptable variation for recovery results at one spiked level is typically on the order of 15-30% RSD. Ideally, one would like to recover 100% of the challenge, but recoveries may be limited to 75%-80%, depending on the sampling conditions and the residue.
ADVANTAGES AND DISADVANTAGES OF SWAB SAMPLING
Advantages:
1. Allows sampling of specific and a defined area of a defined location.
2. Residues that are dried out or are insoluble can be sampled by physical removal.
3. Analysis results of a specific location can be gettable.
4. Allows sampling of areas that are more difficult to clean (i.e. worst cases).
5. Adaptable to wide variety of surfaces.
6. Economical and widely available.
Disadvantages:
1. May contaminate the surface to be sampled (from fibres and solvent).
2. Sampling must include worst case locations so as to represent whole equipment.
3. Difficult to perform swab sampling in inaccessible areas such as complex and large vessels, piping systems and valves etc.
4. Results are dependent upon technique used by the sampler and identification of worst case locations.
IMPORTANCE OF TRAINING ON SWAB SAMPLING:
Swabbing procedure totally depends on the techniques used by the sampler. Training has a very much importance in swab sampling since sampling done by wrong techniques may lead to wrong results. So, the sampler must be trained enough before carrying out any swab sampling. Four keys to consistency in swab sampling training are:
a) emphasis on consistency of wetting the swab head,
b) consistency of the swabbing motion (including overlapping strokes),
c) consistency in applied pressure, and
d) consistency in swabbing of the correct surface area.
Training of the sampler may include training on a particular SOP, the swabbing motion during sampling, labelling of the particular sample, preservation of the sample so as to prevent from any contamination. Training should also be conducted at the sites to be sampled. Training should be conducted repeatedly and as and when required and should be documented.
PROCEDURE OF SWAB SAMPLING:
• Identify the worst case locations of an equipment and define the no. of locations to be sampled. For example, in case of a Rapid mixer granulator (RMG) the worst case locations can be defined as: a) beneath the impeller, inside area of chopper, inner area of the cone, inner area of discharge chute, inner area of bowl etc.
• Identify proper solvent in which the analyte to be sampled is soluble.
• Carry out visual inspection of equipment before swabbing on a surface. The surface should be visually clean.
• Pre-wet the swab in the extracted solvent.
• Swab over the surface at a defined location from a fixed surface area (i.e. 25 cm2 or 100 cm2). Figure-2 shows tools that can be used for swab sampling from a defined area.
• Swab with overlapping pattern. Flip the swab and repeat passing the swab in perpendicular direction. Swab in vertical and horizontal direction as shown in figure-3.
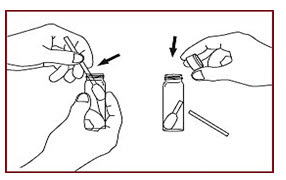
• Snap the swab head at the notch along with narrow edge of swab handle. Allow swab handle to fall into vial (Figure-4).
• Label the sample and transfer it to QC for analysis.
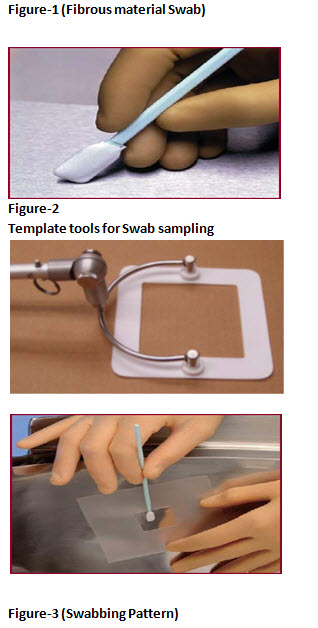
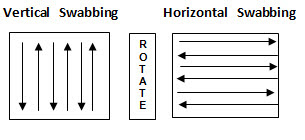
Figure-4 (A notched swab handle simplifies separating the swab head from the handle. Only the Head needs to be extracted for measurement).
RINSE SAMPLING:
Rinse sampling is also known as indirect sampling method since the residue is calculated for overall equipment but not from a particular location of the equipment. The residue amount in equipment after cleaning can also be determined by taking rinse samples. During equipment qualification it should be established that all direct contact parts of the equipment is wetted / reached by the rinsing solvent. After the last cleaning cycle (last rinse), the equipment should be assessed as ‘clean’. In some cases it may be advisable to dry the equipment in order to do a proper assessment. Thereafter, the rinse cycle can be executed, and a sample taken (sampling rinse). The procedure for the rinse cycle and sampling should be well established and described to assure repeatability and comparability (cycle times, temperatures, volumes, etc.). The choice of the rinse solvent should be established during cleaning process, taking into account solubility of the contaminations, and reactivity of the rinse solvent.
For example, water for injection (WFI) should be used as the last rinse for product contact surfaces of an equipment which is utilized in the manufacturing of sterile products and Purified water (PW) should be used as the last rinse for product contact surfaces of an equipment which is utilized in the manufacturing of non-sterile products and sterile products for ophthalmic use. Potable water should not be used in the last rinse of any cleaning procedure for product contact surfaces of equipment because of the presence of varying levels of organic and inorganic residues as well as chlorine.
Rinse sampling can also be performed to demonstrate that residue present on an equipment surface can be effectively reduced and analyzed in rinse solution. Rinse recovery study can be performed by using coupons by the spiking method as done in swab sampling recovery study.
ADVANTAGES AND DISADVANTAGES OF RINSE SAMPLING
Advantages:
a) Larger surface area of an equipment can be sampled.
b) Inaccessible systems or ones that cannot be routinely disassembled can be sampled and evaluated.
Disadvantages:
a) Rinse samples are more susceptible to contamination.
b) Residue or contaminant may not be soluble or may be physically occluded in the equipment.
c) Does not deal with residues that preferentially transfer from one part of the equipment to the next product
IMPORTANCE OF TRAINING ON RINSE SAMPLING:
A major concern involved in rinse sampling is prevention of contamination of the rinse sample. This contamination may come from for example, the sampling port, environment around the sampling port, and/or the operator or sampler. Adequate measures should be taken to prevent contamination such as flushing or cleaning the port prior to taking the rinse sample. Training of the sampler is important in rinse sampling, for instance in grabbing a rinse sample from the final rinse cycle in case of CIP cycle, the timing of sampling process is critical.
Rinse sampling is less dependent on the sampler as compared to that in case of swab sampling. However, training should be conducted repeatedly or as and when required (e.g. in case of any non-conforming result investigation).
PROCEDURE OF RINSE SAMPLING:
• Develop a rinse cycle procedure or SOP as applicable by considering factors such as design, shape, capacity of the equipment, required for washing the equipment and removing previous residue and detergent (if used in the cleaning process). Determine the final rinse solvent quantity. The most common rinse sampling technique is to take a grab sample from the final rinse water during the final rinse of the cleaning process.
• Another option is to fill the entire equipment with water after the cleaning procedure is completed. Then, a bulk sample is taken and analyzed.
• Another option is to utilize a separate CIP sampling rinse of defined volume following the completion of the final process rinse.
REFERENCES:
1. FDA guide to inspections validation of cleaning process, 2004.
2. Technical report no. 49, Points to consider for Biotechnology Cleaning Validation, 2010
3. Technical report no. 29, Points to consider for Cleaning Validation, 2012
4. Cleaning and Cleaning Validation, Volume 2, Paul L. Pluta, 2013.
5. Guidance document Cleaning validation guidelines, Health Canada, 2008.
6. EU guidelines for Good manufacturing practice for Medicinal products for Human and Veterinary use, Eudralex, Volume 4, Annex 15: Qualification and Validation, 2015.
7. Guide to Good Manufacturing Practice for Medicinal Products Part I, PE 009-14, 2018.
8. How to succeed in the search for nothing: Effective swabbing techniques for Cleaning Validation, Howard Siegerman, Wendy Hollands, and Michael Strauss, Texwipe, 2006.
9. Why the Swab matters in Cleaning Validation, Sandeep Kalelkar, 2010.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









