
US Food and Drug Administration (USFDA) has classified the Alembic Pharmaceuticals General Oral Solid Formulation Facility located at Panelav as Voluntary Action Indicated (VAI).

US Food and Drug Administration (USFDA) has classified the Alembic Pharmaceuticals General Oral Solid Formulation Facility located at Panelav as Voluntary Action Indicated (VAI).

U.S. Food and Drug Administration provided an update on its efforts to ensure the availability of alcohol-based sanitizer to help meet the demand for hand sanitizer during the COVID-19 pandemic. As a result of the agency’s significant flexibility, more than 1,500 additional manufacturers have registered with the agency to produce hand sanitizer. At the same time, the agency is addressing safety concerns related to products being sold that are not in line with the FDA’s policy and others being marketed with unproven claims.

Glenmark Pharmaceuticals Inc., USA (Glenmark) has been granted tentativeapproval by the United States Food & Drug Administration (U.S. FDA) for Dapagliflozin and Saxagliptin Tablets, 10 mg/5 mg, the generic version of Qtern®1Tablets, 10 mg/5 mg, of AstraZeneca AB.

U.S. Food and Drug Administration issued a Drug Safety Communication regarding known side effects of hydroxychloroquine and chloroquine, including serious and potentially life-threatening heart rhythm problems, that have been reported with their use for the treatment or prevention of COVID-19, for which they are not approved by the FDA. These risks, which are in the drug labels for their approved uses, may be mitigated when health care professionals closely screen and supervise these patients such as in a hospital setting or a clinical trial, as indicated in the Emergency Use Authorization (EUA) for these drugs to treat COVID-19.

The U.S. Food and Drug Administration has issued warning letters to two companies for illegally selling unapproved products containing cannabidiol (CBD) in ways that violate the Federal Food, Drug and Cosmetic Act (FD&C Act). This action is a continuation of the FDA’s efforts to pursue companies that illegally market CBD products with claims that they can treat medical conditions, including opioid addiction or as an alternative to opioids.

The U.S. Food and Drug Administration authorized the first diagnostic test with a home collection option for COVID-19. Specifically, the FDA re-issued the emergency use authorization (EUA) for the Laboratory Corporation of America (LabCorp) COVID-19 RT-PCR Test to permit testing of samples self-collected by patients at home using LabCorp’s Pixel by LabCorp COVID-19 Test home collection kit.

U.S. Food and Drug Administration granted accelerated approval to Trodelvy (sacituzumab govitecan-hziy) for the treatment of adult patients with triple-negative breast cancer that has spread to other parts of the body. Patients must have received at least two prior therapies before taking Trodelvy.

The Food and Drug Administration (FDA or the Agency) plays a critical role in protecting the United States from threats such as emerging infectious diseases, including the Coronavirus Disease 2019 (COVID-19) pandemic. FDA is committed to providing timely guidance to support response efforts to this pandemic.
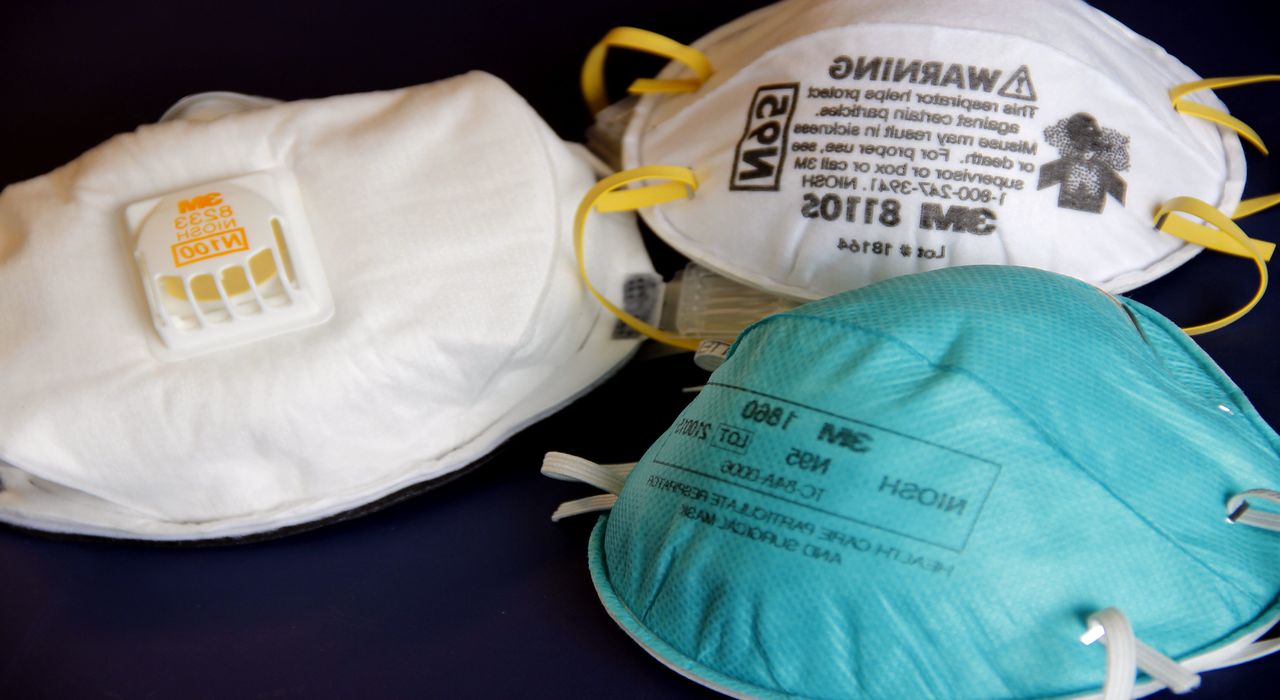
The U.S. Food and Drug Administration issued the second emergency use authorization (EUA) to decontaminate compatible N95 or N95-equivalent respirators for reuse by health care workers in hospital settings. This EUA will support decontamination of approximately 750,000 N95 respirators per day in the U.S.