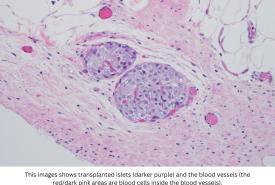
Researchers take step toward development of universal COVID-19 antibodies
SARS-CoV-2, the virus that causes COVID-19 disease, continues to evolve and evade current vaccine and therapeutic interventions. A consortium of scientists at Texas Biomedical Research Institute (Texas Biomed), the University of Alabama at Birmingham (UAB) and Columbia University have developed a promising new human monoclonal antibody that appears a step closer to a universal antibody cocktail that works against all strains of SARS-CoV-2.