Q.1.(b) Cotton effect curves and octant rule, How would you relate stereo chemical features of a compound with them?
Ans.1. (b) Cotton Effect Curve
[adsense:336x280:8701650588]
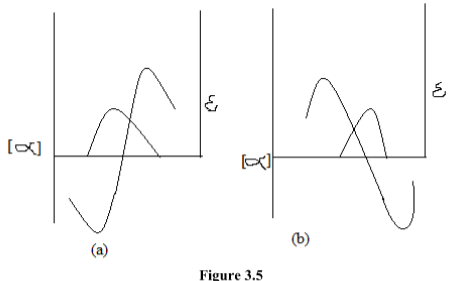
Any medium which is exhibiting circular birefringence may also exhibit circular dichroism. The combination of these two effects in the region in which the optically active absorption bands are observed gives rise to the phenomenon called the cotton effect. And obtain curve known ascotton effect curve. It gives two types of absorption curve that is positive or negative cotton effect curve.
Optically active bands are absorption bands of the chromophores which are either intrinsically asymmetric or which become asymmetric because of the interaction with asymmetric environment.
As an example of the former we may discuss the hexahelicene molecule. In this case the entire molecule acts as one big chromophore.
As an example of latter we consider the carbonyl group which is symmetric but becomes optically active in an asymmetric environment. Hence the carbonyl group in acetone is optically inactive because it is a symmetric molecule environment. The same carbonyl group in 3-methylcyclohexanone, however, becomes optically active because there is an asymmetric carbon atom present. It is expected that such induced optical activity would be appreciably smaller than the case in which the whole molecule acts as chromophore. This is indeed true in general.
Both the absorption and circular dichroism phenomenon are having their origin in the charge displacements which are caused by the interaction with the electromagnetic radiation. As a result there will be induced electric and magnetic dipoles. We define the rotational strength Rkof a chromophore for the k electronic transition as follows :
Rk=ρµcosθ
Where ρ and µ refer to the electric and magnetic transition moments and θ refer to the angle between the directions of these two moments. For a molecule having either a centre of inversion or a reflation plane of symmetry, we can have any of the following three situations:
ρ ≠ 0 but µ = 0 electric dipole allowed but magnetic dipole forbidden transition
ρ ≠ 0 but µ = 0 electric dipole allowed but electric dipole forbidden transition
ρ┴ µ cosθ = 0 in all cases, Rk = 0 and the molecule is optically inactive.
Octant Rule
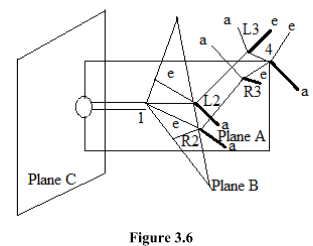
This is very useful empirical rule in predicting the sign and magnitude of the cotton effect. This rule applies only to the substitute cyclohexanones. The cyclohexanone molecule is divided into eight octants by three planes A, Band C shown in figure1.4.
Plane A passes carbon atoms 1 and 4; the substituents attached to carbon atoms 4 thus lie in this plane.
Plane B encompasses carbon atoms 1, L2 and R2 where L and R denotes left and right from the observer’s point of view. The substituents in the equatorial positions at L2 and R2 are practically in this plane.
Plane C bisects the carbonyl group and is perpendicular to plane A and B. thus plane A and B produce four octants and plane C produce four more. The midpoint of the C = O bond is chosen to be the origin of the coordinate system.
The octant rule may be stated that substituents in the lower left and far upper right octants make a negative contribution to the cotton effect, substituents in the far lower right and far upper left make a positive contribution; substituents in any of the three planes do not make contribution.
As rarely do substituents bend over the carbonyl group towards the oxygen atom beyond, the four octants in the front of plane C are usually vacant and will not concern us. Thus we shall consider only the four octants defined by planes A and B. as a simple illustration of this rule let us consider 3-methylcyclohexanone molecule.
Relation of Stereochemical features of compounds with them
The sign of Cotton effect gives information about the stereochemistry in the nearby environment of the chromophore, i.e., the carbonyl group (n→ π* absorption of the carbonyl group around 280 nm) acts as a probe of the chirality of its environment. Consider the following points
(i) When two compounds display curves of the same sign and shape, the stereochemical features near the chromophore are same.
(ii) When two compounds show cotton effects of opposite sign then the stereochemical features near the chromophore are mirror image type.
(iii) The compound (II and III,) are not enantiomers (these are different compounds), however, their ORD curves have an almost mirror image relationship. Thus the compounds have an object mirror image i.e., enantiomeric stereochemistry of the groups in the immediate vicinity of the carbonyl group and this is A/B ring junction.









