Indian pharmaceutical industry is the 3rd largest in the world by volume and is USD 40 billion in terms of value. The country contributes 3.5% of total drugs and medicines exported globally. India exports pharmaceuticals to more than 200 countries and territories including highly regulated markets such as the USA, The UK, European Union, Canada etc. India has complete ecosystem for development and manufacturing of pharmaceuticals with companies having state of the art facilities, highly skilled/technical manpower. The country also has a number of renowned pharmaceutical educational and research institutes and a robust ecosystem of allied industries.
At present a major component of Indian exports are low value generic drugs while a large proportion of the demand for patented drugs is met through imports. This is because the Indian Pharmaceutical sector lacks in high value production along with world class pharma R&D. In order to incentivize the global and domestic players to enhance investment and production in these product categories, a well-designed and suitably targeted intervention is required to incentivise specific high value goods such as biopharmaceuticals, complex generic drugs, patented drugs or drugs nearing patent expiry, cell based or gene therapy drugs.
Further, looking to the increasing imperative of drug security, continued support to domestic production capability in APIs/KSMs would ensure higher resilience of the Indian pharmaceutical industry to external shocks. These initiatives have the potential to contribute significantly to achieving higher objective of affordable healthcare in the country and globally on a sustained basis.
Objective
The objective of the scheme is to enhance India’s manufacturing capabilities by increasing investment and production in the sector and contributing to product diversification to high value goods in the pharmaceutical sector. One of the further objectives of the scheme is to create global champions out of India who have the potential to grow in size and scale using cutting edge technology and thereby penetrate the global value chains.
Salient Features of the Scheme:
Target Groups: The manufacturers of pharmaceutical goods registered in India will be grouped based on their Global Manufacturing Revenue (GMR) to ensure wider applicability of the scheme across the pharmaceutical industry and at the same time meet the objectives of the scheme. The qualifying criteria for the three groups of applicants will be as follows-
(a) Group A : Applicants having Global Manufacturing Revenue (FY 2019-20) of pharmaceutical goods more than or equal to Rs 5,000 crore.
(b) Group B : Applicants having Global Manufacturing Revenue (FY 2019-20) of pharmaceutical goods between Rs 500 (inclusive) crore and Rs 5,000 crore.
(c) Group C : Applicants having Global Manufacturing Revenue (FY 2019-20) of pharmaceutical goods less than Rs 500 crore. Within this group, a sub-group for MSME industry will be made given their specific challenges and circumstances.
Quantum of Incentive : The total quantum of incentive (inclusive of administrative expenditure) under the scheme is about Rs 15,000 crore. The incentive allocation among the Target
Groups is as follows-
(a) Group A: Rs 11,000 crore.
(b) Group B: Rs 2,250 crore.
(c) Group C: Rs 1,750 crore.
Rate of Incentive : The rate of incentive on incremental sales (over base year) of pharmaceutical goods covered under Category 1 & 2 will be 10% for FY 2022-23 to FY 2025-26, 8% for 2026-27 and 6% for 2027-28.
The rate of incentive on incremental sales (over base year) of for pharmaceutical goods covered under Category-3 will be 5% for FY 2022-23 to FY 2025-26, 4% for 2026-27 and 3% for 2027-28.
Base Year : Financial Year 2019-20 shall be treated as the base year for computation of incremental sales of manufactured goods
Category of Goods : The scheme shall cover pharmaceutical goods under three (03) categories as mentioned below:-
(I) Category 1
1. Bio-pharmaceuticals
2. Complex generic drugs
3. Patented drugs or drugs nearing patent expiry
4. Cell based or gene therapy drugs
5. Orphan drugs
6. Special empty capsules like HPMC, Pullulan, enteric etc.
7. Complex excipients
8. Phyto-pharmaceuticals
9. Other drugs as approved
(II) Category 2
1. Active Pharmaceutical Ingredients / Key Starting materials / Drug Intermediates
(III) Category 3 (Drugs not covered under Category 1 and Category 2)
1. Repurposed drugs
2. Auto immune drugs, anti-cancer drugs, anti-diabetic drugs, anti- infective drugs, cardiovascular drugs, psychotropic drugs and anti- retroviral drugs
3. In-vitro diagnostic devices
4. Other drugs as approved
5. Other drugs not manufactured in India.
Selection of participants: The applicants will be selected based on pre-defined objective criteria to assess their experience, capacity to grow in scale and innovate. The selection criteria shall be elaborated in the scheme guidelines.
Eligibility for incentive: The selected participants in the scheme will be eligible for incentives on incremental sales of pharmaceutical goods based on yearly threshold criteria of minimum cumulative investment and minimum percentage growth in sales as mentioned below.
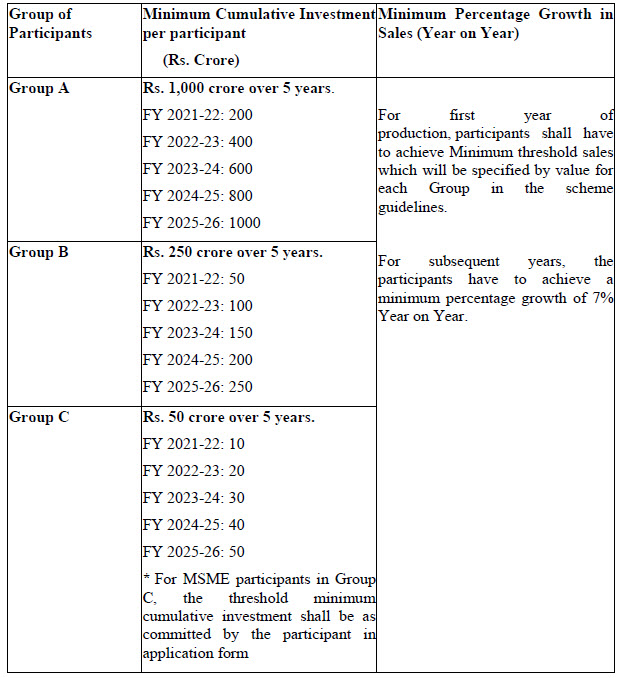
Tenure of the Scheme : The duration of the scheme will be from FY 2020-21 to FY 2028- 29. This will include the period for processing of applications (FY 2020-21), optional gestation period of one year (FY 2021-22), incentive for 6 years and FY 2028-29 for disbursal of incentive for sales of FY 2027-28.
Incentive Outlay: Total financial outlay of the scheme is Rs. 15,000 crore. The annual incentive outlay is estimated based on projected incremental sales of the identified pharmaceutical goods by the selected participants. The incentives will be paid for a maximum period of 6 years for each participant. Participants may avail of up to one-year gestation period from the date of approval.
Incentive ceiling : Incentive per participant will be subject to ceilings which will be specified in the scheme guidelines.
Empowered Group of Secretaries (EGoS): The Empowered Group of Secretaries (EGoS) comprising of Cabinet Secretary (Chairperson), CEO NITI Aayog (Member), Secretary DPIIT (Member Convenor), Secretary DoC (Member), Secretary DoR (Member), Secretary DEA (Member) and Secretary DoP shall undertake periodic review of the outgo under the scheme, ensure uniformity with other PLI schemes and take appropriate action to ensure that the expenditure is within the prescribed outlay.
Technical Committee : A Technical Committee (TC) of 5-7 members will be formed with representative from CDSCO, experts from industry and academia. The role of the TC along with the scope and manner of its functioning shall be laid down in the scheme guidelines.
Project Management Agency : The Scheme shall be implemented by the Department of Pharmaceuticals through a Project Management Agency (PMA) that will be responsible for providing secretarial, managerial and implementation support and carry out other responsibilities as assigned by DoP within the framework of scheme and guidelines thereof.
Approval and Disbursement Process:
(a) Application under the Scheme can be made by any manufacturer registered in India.
(b) An application, complete in all aspects, will have to be submitted before the due date. Acknowledgement will be issued after initial scrutiny of the application.
(c) The applicants will be appraised and considered for approval, based on predefined selection criteria.
(d) The incentives shall be released to the selected participants under the scheme who meet the annual threshold criteria of minimum cumulative investment and minimum growth in sales and if disbursement claims are found to be in order.
(e) Timely disbursal of incentives by the project Management Agency will be monitored by DoP and reviewed by the EGoS.
(f) The incentive will be disbursed on incremental sales for a maximum period of 6 years for each participant.
(g) The progress in approval of applications and disbursal of incentive shall be monitored on an ongoing basis against the monitoring framework to be specified in the guidelines.









