ABOUT AUTHORS:
Saxena Vaishali*, Bhale Shweta, Dantoriya Sanjay, Mathariya Arun k., Mahajan S.C., Bhandari Govind
Mahakal Institute of Pharmaceutical studies,
Ujjain (m.p)
*vaishali.saxena0390@gmail.com
ABSTRACT:
Pain management is important for ongoing pain control, especially if you suffer with long-term or chronic pain. NSAIDs or nonsteroidal anti-inflammatory drugs are among the most common pain relief medicines in the world. Every day more than 30 million peoples use them to soothe headaches, sprains, arthritis symptoms, and other daily discomforts, according to the American Gastroenterological Association. Propionic acid derivatives are widely regarded as the drugs of first choice in the management of patients with inflammatory joint disease because they are the class of NSAIDs with the lowest incidence of side effects. Zaltoprofen (ZLT) is a propionic acid derivative of non steroidal anti inflammatory class, which has excellent effect on post-surgery or post trauma chronic inflammation of the drug. So, Zaltoprofen may serve as a potent and superior analgesic for the treatment of pain. Zaltoprofen has the dose of 80 mg three times a day which reduce patient compliance and used in the treatment of rheumatoid arthritis, osteoarthritis, and other chronic inflammatory Pain conditions. ZLT has recently been reported to cause potent inhibition of cyclooxygenase-2 with fewer side effects on the gastro-intestinal tract than other non steroidal anti inflammatory drugs.
REFERENCE ID: PHARMATUTOR-ART-1858
INTODUCTION:
Pain is an unpleasant feeling often caused by intense or damaging stimuli, such as stubbing a toe, burning a finger, putting alcohol on a cut, and bumping the "funny bone."(1, 2)The International Association for the Study of Pain's widely used definition states: "Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage".(3) On a basic level, pain is the result of an electrical signal being sent from your nerves to your brain. But the process is not only electrical. When you get injured, say with a sprain, he damaged tissue releases chemicals called prostaglandins, which are like hormones. These prostaglandins cause the tissue to swell. They also amplify the electrical signal coming from the nerves. Basically, they increase the pain you feel. (4)
NSAIDs: Non steroidal anti-inflammatory drugs (NSAIDs) are among the most widely used medication in clinical practice because of their well established efficacy in reducing pain and inflammation. NSAIDs are a necessary choice in pain management, because of the integrated role of the COX pathway in the process of inflammation. NSAIDs are widely used in both the clinical setting like acute or chronic pain setting. (5, 6)
CLASSIFICATION OF NONSTEROIDAL ANALGESICS: Table 1 ( 5, 7)
ZALTOPROFEN:
Description- Zaltoprofen is an effective NSAIDs, which is came from Japan. ZLT, 2-(10, 11-dihydro-10-oxodibenzo [b, f] thiepin-2-yl) pro-pionic acid is a potent non-steroidal anti-inflammatory drug (NSAID).8 Propionic acid derivatives are widely regarded as the drugs of first choice in the management of patients with inflammatory joint disease because they are the class of NSAIDs with the lowest incidence of side effects. Ibuprofen was “the first of the propionic” launched in 1969. Since then, Ibuprofen proved to be effective as a prescription drug in a range of painful non rheumatic condition and on the basis of its good safety. It has been used clinically for treatment of post-operative pain and record was approved as an OTC analgesic in 1983 in the United Kingdom and in 1984in the USA. In India, the drug is widely used in clinical practice. ZLT belongs to this class of NSAIDs. (5,9,10) It has been used clinically for treatment of post-operative pain and low back pain for more than ten years. Zaltoprofen is a preferential COX-2 inhibitor3 and selectively inhibits prostaglandin E2 (PGE2) production at inflammatory sites.2 and to induce apoptosis in a variety of cell lines. Zaltoprofen is a unique compound that also has anti-bradykinin activity. Its analgesic effects may be a result of inhibition of bradykinin B2 receptor-mediated bradykinin responses not only of cyclooxygenases but also of bradykinin-induced 12-lipoxygenase inhibitors.8 (FIG.1)
WHY ZALTOPROFEN IS SUPERIOR AS COMPARE TO OTHER NSAIDs?
There are two type of COX enzyme- COX-1 constitutive (responcible for normal GI functions) and COX-2 inducible (responcible for pain). ZLT is the preferential COX-2 inhibitor. ZLT inhibit COX-2 inducible enzyme, while other NSAIDs inhibit both COX-1 and COX-2 enzyme( constitutive and inducible) and damage the normal body function. So, ZLT does not interfere with normal GI functions.Zaltoprfen shows excellent GI safety as compare to other NSAIDs.12 (Fig.2)
DRUG PROFILE:13 Table2
GEOMETRY:14
- Dreiding energy = 65.56 kcal/mol
- Volume = 257.85 Å3
- Minimal projection area = 44.27 Å2
- Min z length = 13.85 Å
- Maximal projection area = 82.63 Å2
- Max z length = 8.75 Å (fig.3)
MODE OF ACTION: Zaltoprofen is a nonsteroidal anti-inflammatory drug that exhibits anti-inflammatory, analgesic and antipyretic activities. It is a COX-2 preferential inhibitor. The main mechanism of zaltoprofen is prostaglandin biosynthesis inhibitory action due to the COX inhibition in the arachidonic acid metabolism system. Besides this, membrane stabilizing action such as leukocyte migration inhibitory action and lysosomal enzyme inhibitory action are also observed with zaltoprofen.(fig.4) Experimental studies have shown that Prostaglandin biosynthesis inhibitory action in the stomach tissue is weaker with ZLT than in case of indomethacin. ZLT was shown to have more powerful inhibitory effect to bradykinin-nociceptor than other NSAIDs. (5, 15)
ZALTOPROFEN NSAID WITH NOVEL ANTIBRADYKININ PROPERTY: Zaltoprofen specially blocks the nociceptive response induced by bradykinin. It has a strong inhibitory effect on bradykinin induced swelling and pain.(5,16) Bradykinin and prostaglandin are among the most potent autacoids involved in vascular, inflammatory and pain process. Mixture of these products released due to tissue damage is known as “Inflammatory Soup”. ZLT exerts its analgesic effect by blocking the B2 receptor mediated signalling pathway on primary sensory neuron. (17) ZLT has a strong inhibitory effect on bradykinin induced pain at 5-20mg/kg. (2,18) (fig.5)
PHARMACOKINETIC: Zaltoprofen is well absorbed on oral administration, with more than 82% absorption. ZLT is more than 98% bound to plasma proteins. No accumulation is observed after repeated dose of ZLT. It is predominantly metabolized by CYP2C9 and UGT2B7 in liver. ZLT is also biotransformed to S-oxide zaltoprofen (M-2), 10-hydroxy zaltoprofen (M-3) and S-oxide-10-hydroxy-zaltoprofen (M-5) in humans, and conjugate of M-2 and M-3 are excreted in urine, although urinary level of each of these metabolites account for less than 10% of the dose. After oral administration, 62% of the dose is excreted as conjugate in the urine and only 3% is excreted as unchanged compound by this route. (table3) There is a biphasic reduction in plasma concentration. (T1/2α around 0.9hours and T1/2β around 9hours). (5, 10)
PHARMACODYNAMIC: Zaltoprofen is a NSAID with powerful anti-inflammatory and analgesic effects on inflammatory pain. It is a preferential COX-2inhibitor; it selectively inhibits PGE2 production at site of inflammation. It exhibits a powerful anti-inflammatory effect with ED50, 1-5mg/kg, p.o.dose.(5,20) COX activity for various NSAIDs drugs. Higher IC50 ratio of COX-1/COX-2 indicates selectively for COX-2. (5,16) (Table4)
MICROMERITIC PROPERTIES:
Surface morphology:The results of surface morphology studies were shown in Scanning Electron Microscopy (SEM). The parent zaltoprofen crystals were in the form of fine needle. This long-needle form of zaltoprofen leads to very poor flow and compressional difficulties.(21, 22) (fig.6)
IR spectroscopy:IR spectroscopy studied the possible interaction between the drug and the carrier. (fig.7)The interaction often leads to identifiable changes in the IR profile and melting point of drug.(21) The principal IR peaks of pure zaltoprofen were shown in Table5.
Differential Scanning Calorimetry Study: The DSC thermograms of pure zaltoprofen were shown in Fig.8. Pure zaltoprofen showed a sharp endotherm at 140.81oC corresponding to its melting point. (21, 22)
FT-IR Spectrum: Fourier-transform infrared (FT-IR) spectra were obtained using an FT-IR spectrometer. The zaltoprofen pure drug was mixed with KBr. The KBr discs were prepared by compressing the powders at a pressure of 5 tons for 5 min in a hydraulic press, which were analysed. (fig.9)All spectra acquired scans between 400 to 4000 cm-1.(23)
High-performance liquid chromatography study: A high-performance liquid chromatography (HPLC) methodology useful for the quantification of ZLT in human plasma samples was reported. An accurately weighed sample (50 mg) of ZLT reference standard was transferred to a 100 mL volumetric flask and dissolved in acetonitrile to make a stock solution of 0.5 mg mL−1. Aliquots from the stock solution were diluted with the diluent to give the solutions in the concentration range 10-140 μg mL−1. (fig.10)The solutions were injected in HPLC and area was measured for each solution. The calibration curve was obtained by plotting peak area on ordinate against drug concentration on abscissa.24
X-Ray Diffraction Study: The atomic planes of a crystal cause an incident beam of X-rays to interfere with one another as they leave the crystal. The phenomenon is called X-ray diffraction. X-ray diffraction study is used for measure the average spacings between layers or rows of atoms, determine the orientation of a single crystal or grain, find the crystal structure of an unknown material, measure the size, shape and internal stress of small crystalline region.25 (fig.11)
Animal studies:The Pharmacokinetics of Zaltoprofen was studied after a Single Intravenous and Oral Administration in Rats. Zaltoprofen was administered intravenously (0.3 mg/kg) and orally (1 mg/kg) to male Wistar rats as a solution. At predetermined time points, plasma was collected from rats and analysed for zaltoprofen by HPLC. Data were analysed using noncompartmental analysis model. After intravenous dosing, the plasma concentration of zaltoprofen declined monoexponentially with a terminal half-life of 2.82 h. Zaltoprofen was absorbed rapidly after oral dosing with 84% bioavailability. After oral dosing (1 mg/kg), the peak plasma concentration of zaltoprofen was 2516 ± 319 ng/mL.26 (Table 6)
INDICATION:
Zaltoprofen is indicated for its analgesic and anti-inflammatory activities against following diseases and symptoms-
- Rheumatoid arthritis: Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by joint swelling, joint tenderness, and destruction of synovial joints, leading to severe disability and premature mortality.27 Zaltoprofen is highly safe and efficient drug for the treatment of chronic rheumatoid arthritis.5
- Osteoarthritis: Knee osteoarthritis is a degenerative disease of the knee joint. It is more common in people older than 40 years. Women have greater chance to be affected.Some of the signs and symptoms associated with knee osteoarthritis include: Pain, Stiffness, Decreasing range of motion, Muscle weakness and atrophy (particularly of quadriceps femoris, muscle on front of thigh) due to inactivity or stiffness, Crepitus, Effusion-increase in quantity of synovial fluid leading to swelling.28 Zaltoprofen shows excellent effect in treatment of knee osteoarthritis by inhibiting B2 receptor.5
- Low back pain: Zaltoprofen has been used clinically for the treatment of low back pain for more than decade internationally. Zaltoprofen possesses a desirable property of reduction of incisional pain and subsequent inflammatory pain. Zaltoprofen exerts the pre-emptive analgesic effect by its Antibradykinin property.5
- Post operative pain: Pain that occur after operation. Postoperative pain is defined as an acute or chronic pain that is experienced after an operation or surgical procedure.29 Zaltoprofen shows significant improvement in post operative pain by blocking the bradykinin and prostaglandin pathway.5
- Periarthritis scapulohumeralis: Shoulder bursitis/tendinitis is a common overuse injury in sports where the arm is used in an overhead motion (e.g., swimming, baseball). Originally described (1872) as periarthritis scapulohumeralis its diagnosis and treatment were developed by Neer – 1972. The pain-usually felt at the tip of the shoulder and referred down the deltoid muscle into the upper arm-occurs when the arm is lifted overhead or twisted.30 Zaltoprofen shows remarkable improvement in periarthritis scapulohumeralis.5
- Cervico-omo-brachial syndrome: Cervical Brachial Syndrome is primarily a stretching of the Brachial plexus. This is generally caused by a breakdown of the normal mechanical function of the neck and is characterized by the sharp, radiating pain in the neck, shoulder arm and/or hand.31 Zaltoprofen shows significant improvement in cervico-omo-brachial syndrome by blocking the bradykinin and prostaglandin pathway.5
- Musculoskeletal joint disorder: Musculoskeletal pain affects the bones, muscles, ligaments, tendons, and nerves. It can be acute (having a rapid onset with severe symptoms) or chronic (long-lasting). Musculoskeletal pain can be localized in one area, or widespread. It is most often caused by an injury to the bones, joints, muscles, tendons, ligaments, or nerves. This can be caused by jerking movements, car accidents, falls, fractures, sprains, dislocations, and direct blows to the muscle.32
- Limited shoulder movement in breast cancer: The painful disorders that contribute to limited shoulder movement in breast cancer survivors have various etiologies, including rotator cuff tendonitis, adhesive capsulitis, axillary tightness, lymphatic cording, and phlebitis. Unless the pain is resolved, PT will not be effective in extending the joint range of motion (ROM). After taking zaltoprofen, the active shoulder movements in each direction were improved without ROM exercises.33
- Dental inflammation:Dental inflammation: Usually due to inflammation of the tooth pulp which contains blood vessels and nerve fibers which is responsible for pain and inflammation.34 Pulpitis is the medical symptom in which the dental pulp becomes inflamed.35
It is also indicated for its analgesic and anti-inflammatory activities after surgery, injury and after tooth extraction.5
CONTRAINDICATION:
Zaltoprofen is contraindicated in patients with;
- Previous history of hypersensitivity to any of the components of the medicine.
- Active peptic ulcer or GI bleeding or history of peptic ulcer disease.
- Severe blood abnormalities (dysemia).
- Severe hepatic impairment.
- Severe renal impairment.
- Severe heart failure or cardiac insufficiency.
- Chronic ulcerative colitis or Crohn’s disease
- Patients who have experienced asthma, urticaria, or other allergic type reactions after taking aspirin or other NSAIDs.5
ZALTOPROFEN TOLERABILITY:
Zaltoprofen is well tolerated. Adverse effects of ZLT include the following:
Gastrointestinal- Nausea, vomiting, constipation, dry mouth, loss of apatite.
Neurologic-Drowsiness, dizziness, numbness.
Hypersensitivity-Photosensitivity, itching.
Hematologic-erythrocytopenia, thrombocytosis.
Renal-Blood in urine rises in creatinine.
Hepatic- Rise in AST (GOT), rise in ALT (GPT).
Other- Increased urinary frequency, pain on urination, fever, urinary disturbance. (5, 36)
DRUG INTERACTION:
Quinolone antibacterials- Concomitant use of antibacterials with zaltoprofen may trigger convulsion. The dose may have to be adjusted in such cases.
Coumarin anticoagulant agent- the dose may have to be adjusted as there may be intensification in the anticoagulant action.
Sulfonylurea antidiabetic agents- The dose may have to be adjusted as there may be an intensification in the hypoglycaemic action.
Lithium- The dose of lithium may have to be adjusted as there may be intensification in the lithium action. (5, 36)
OVERDOSAGE: No human data available on the consequences of zaltoprofen overdosage. The therapeutic measures to be taken are: absorption should be prevented, as soon as possible after overdosage by means of gastric lavage and treatment with activated charcoal; supportive and systematic treatment should be given for complications such as hypotension, renal failure, convulsion, gastrointestinal irritation and respiratory depression, specific therapies such as forced diuresis, dialysis or haemoperfusion are probably of no help in eliminating NSAIDs due to their high rate of protein binding and extensive metabolism.5
HOW SUPPLIED: Zaltokin (Zaltoprofen tablet) is available as:
- 80mg, thrice a day, oral administration.
- Alu – Alu pack of 10 tablets.
- Store in cool, dark, dry place. Keep out of reach of children.5
PUBLISHED RESEARCHES OF ZALTOPROFEN:
Oral Controlled Porosity Osmotic Pump Tablet of Zaltoprofen: Controlled Porosity osmotic pump of Zaltoprofen that can deliver Zaltoprofen for 12 hr. An inclusion complex was prepared by kneading method using HP-β-CD in order to increase solubility of the poorly water soluble drug. CPOP tablet containing Zaltoprofen were prepared by direct compression method by using various osmotic agent like sodium bicarbonate, sodium chloride, mannitol and potassium carbonate. Cellulose acetate, Sorbitol and Poly Ethylene Glycol 400 were selected for coating materials, and acetone: methanol (65:35) co-solvent was employed as the coating medium with 3% and 5% weight gain. CPOP which was prepared by the use of NaHCO3 in 75 mg and 3% coating weight gain has the maximum % drug release of 98.08% in dissolution study.23
Zaltoprofen Gel: Zaltoprofen percutaneous forms (GEL) are not only conducive to arthritis, pain and inflammation treatment, but also to overcome caused by the oral side effects. Carbomer concentration of propylene glycol, Azone had greater influence on permeability of ZTPF gel in vitro; the best prescription optimized with orthogonal design contained 0.75%Carbomer,1.0%Azone;15%propylene glycol. Zatoprofen gel was proved non-toxic and irritating through the acute toxicity tests, skin irritation and allergic test. Zatoprofen gel could increase the local concentration, with a high degree of security and good convenience, besides a reduced dosage also could get the same treatment efficiency, reduce the gastrointestinal tract and the body of the organizations.37
Zaltoprofen spherical agglomerates: Zaltoprofen spherical agglomerates prepared with poly ethylene glycol, which is hydrophilic polymer by using simple spherical agglomeration technique for enhancing micromeritic properties and dissolution rate. The agglomerates have shown improved in-vitro drug release performance comparable with untreated zaltoprofen.21
CONCLUSION: Zaltoprofen has been speculated to have unique pharmacological activities in inhibiting BK-induced nociception, as well as NSAID action, which is known as inhibitory action of COX. Zaltoprofen shows excellent inhibition of bradykinin pathway, prostaglandin pathway and leukotrins pathway. So, we found that Zaltoprofen is powerful pain producing substance inhibitor.
FIGURE
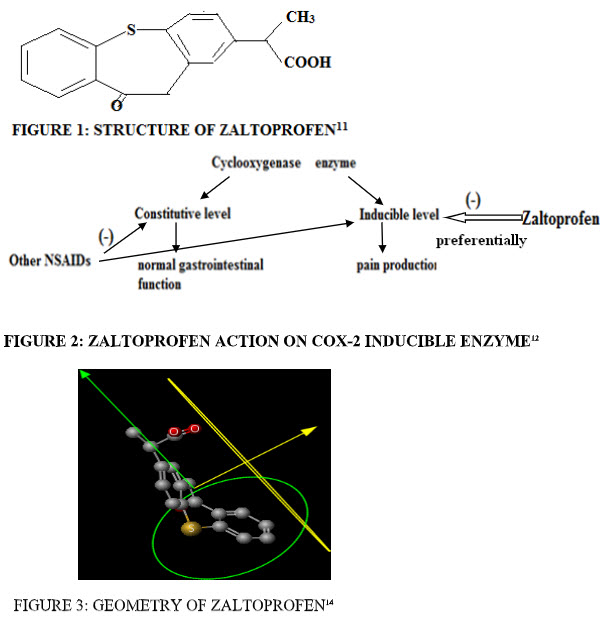
FIGURE 2: ZALTOPROFEN ACTION ON COX-2 INDUCIBLE ENZYME12| FIGURE 3: GEOMETRY OF ZALTOPROFEN14
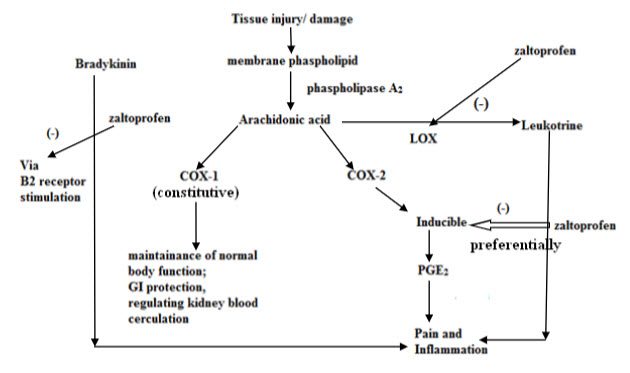
FIGURE4: TRIPLE MODE OF ACTION OF ZALTOPROFEN5
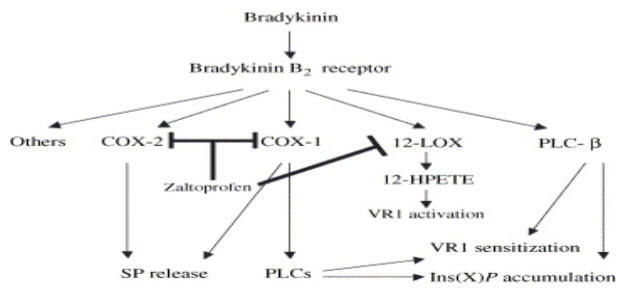
FIGURE 5: Possible mechanism ZLT inhibits bradykinin induced response.
12-HPETE=12-(S)-hydroperxyeicosatetraenoic acid; 12-LOX=12-lipoxygenase; PLCs=phospholipase Cs; SP=substance P; VR1=vanolloid receptor; Ins (X) P=inositol phosphate.(15,16)
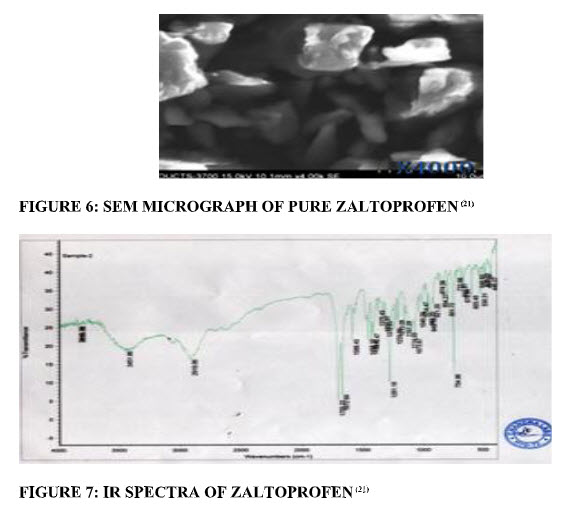
FIGURE 6: SEM MICROGRAPH OF PURE ZALTOPROFEN(21) | FIGURE 7: IR SPECTRA OF ZALTOPROFEN(21)
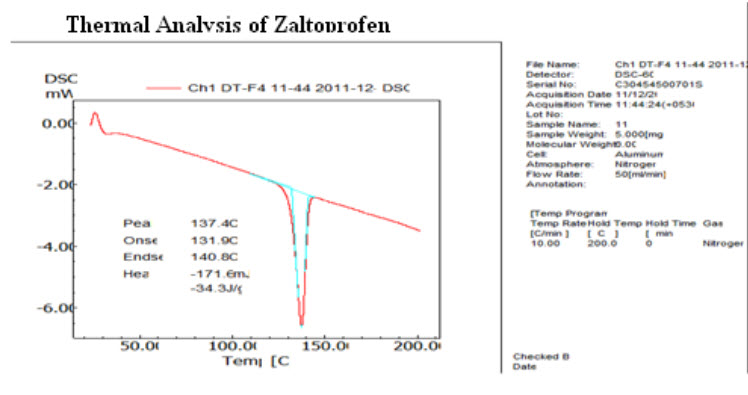
FIGURE 8: DSC OF PURE ZALTOPROFEN (21)
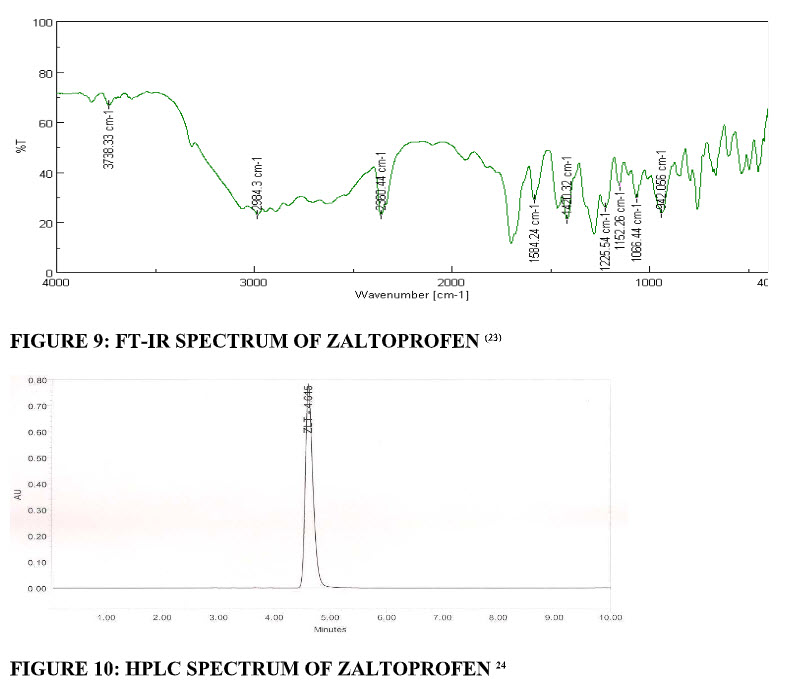
FIGURE 9: FT-IR SPECTRUM OF ZALTOPROFEN (23)| FIGURE 10: HPLC SPECTRUM OF ZALTOPROFEN 24
FIGURE 11: X-RAY DIFFRACTION OF PURE ZALTOPROFEN DRUG 21
TABLE
TABLE 1: CLASSIFICATION OF NSAIDS
|
S.NO. |
CLASS |
DRUGS |
|
1 |
Salicylates |
Aspirin, Methyl salicylate |
|
2 |
Para-aminophenol derivative |
Acetaminophen |
|
3 |
Acetic acid derivative |
Indomethacin, Diclofenac |
|
4 |
N-Arylanthranillic acids |
Mefenamic acid |
|
5 |
Pyrazolidine derivative |
Phenylbutazone, Oxyphenbutazone |
|
6 |
Propionic acid derivative |
Ibuprofen, Zaltoprofen |
|
7 |
Enolic acid derivative |
Piroxicam |
|
8 |
COX-2 selective inhibitor |
Celecoxib, Refecoxib |
TABLE2: DRUG PROFILE OF ZALTOPROFEN
|
PARAMETER |
ZALTOPROFEN |
|
Molecular formula |
C17H14O3S |
|
Molecular weight |
298.36 |
|
IUPAC name |
(2RS)-2-(10-Oxo-10,11-dihydrodibenzo[b,f] thiepin- 2-yl)propanoic acid |
|
Dose |
80 mg, TID |
|
Percent Composition |
C 68.43%, H 4.73%, O 16.09%, S 10.75% |
|
Standard |
Zaltoprofen, when dried, contains not less than 99.0%and not more than 101.0% of C17H14O3S. |
|
appearance |
Zaltoprofen occurs as white to light yellow, Crystals or crystalline powder. Tasteless, odourless. |
|
Therapeutic-Category |
Anti-inflammatory |
|
Solubility |
Freely sol in acetone, chloroform; sol in methanol; slightly sol in ethanol, benzene. Practically insoluble in water, cyclohexane |
|
Light effect |
It is gradually decomposed by light |
|
Derivative Type |
(S)-form |
|
Melting point |
135 – 139oC |
|
Loss on drying |
Not more than 0.5% (1 g, 105oC, 4hours) |
|
Residue on ignition |
Not more than 0.1% (1 g) |
|
Containers and storage |
Tight containers and store in cool, dry, dark place. |
|
Optical rotation
|
A solution of Zaltoprofen in acetone (1 in 10) shows no optical rotation. |
|
Usage |
Anti-inflammatory activity resides in (S)-enantiomer |
TABLE 3: PHARMACOKINETICS PARAMETER (5, 19)
|
PARAMETER |
ZALTOPROFEN |
|
Cmax (μg/ml) |
11.33± 4.01 |
|
AUC 0-24 (μg/ml) |
31.51± 10.01 |
|
Tmax (h) |
1.6 ±0.6 |
|
T1/2 |
6.8 ± 2.1 |
TABLE 4: COX ACTIVITY OF DIFFERENT NSAIDS17
|
NSAIDS |
COX-1 IC50(μm) |
COX-2 IC50(μm) |
COX-1/COX-2 Ratio |
|
Aspirin |
1.7 |
7.5 |
0.23 |
|
Indomethacin |
0.013 |
0.044 |
0.30 |
|
Ibuprofen |
3.0 |
3.5 |
0.86 |
|
Diclofenac |
0.076 |
0.026 |
2.92 |
|
Zaltoprofen |
1.3 |
0.34 |
3.82 |
|
Etodolac |
100 |
53 |
1.89 |
|
Ketoprofen |
0.11 |
0.88 |
0.13 |
TABLE 5: IR PEAK OF PURE ZALTOPROFEN DRUG(21)
|
Samples |
Major peaks (Wave numbers), cm-1 |
|
Pure Zaltoprofen |
1705.08, 1672.58, 1588.29, 1458.11, 1014.06, 603..34 |
TABLE 6: ANIMAL STUDY OF ZALTOPROFEN BY DIFFERENT ROUTE26
|
Parameter
|
Oral (1 mg/kg, n=3) |
Intravenous (0.3 mg/kg, n=3) |
|
Cmax (ng/mL) |
2516 ± 319 |
2674 ± 2.38 |
|
Tmax (h) |
0.63 ± 0.53 |
0.03 ± 0.0 |
|
AUC0-t (ng.h/mL) |
11335 ± 29 |
4039 ± 356 |
|
AUC0-inf (ng.h/mL) |
12051 ± 120 |
4242 ± 381 |
|
T1/2 (h) |
9.63 ± 1.70 |
2.82 ± 1.0 |
|
Vd (mL/kg) |
1154 ± 214 |
287.5 ± 100 |
|
Cl (mL/h/kg) |
83 ± 0.8 |
71.1 ± 6.1 |
|
MRT last (h) |
6.24 ± 1.89 |
2.02 ± 0.0 |
REFERENCES:
1. en.wikipedia.org/wiki/Pain.
2.The examples represent respectively the three classes of nociceptive pain - mechanical, thermal and chemical – and neuropathic pain.
3.International Association for the Study of Pain: Pain Definitions [cited 10 Sep 2011]. "Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage" Derived from Bonica JJ. The need of taxonomy. Pain. 1979; 6(3):247–8. Doi :10.1016/0304-3959(79)90046-0. PMID 460931.
4. arthritis.webmd.com/features/pain-relief-how-nsaids-work
5.ACTIVA, A division of Ipca laboratory, product monograph of Zaltokin brand of Zaltoprofen, 1-34.
6.Ong CK et al, Clin Med Res, 2007, Mar; 5(1), 19-34.
7.Goodman & Gilman’s, the pharmacological basis of therapeutics, 11th edition.
8.T. Manish Kumar et al., Development and validation of HPLC-UV method for the estimation of zaltoprofen in human plasma, Journal of Pharmacy Research 2011, 4(10), 3753-3755.
9.Nuki G et al, Br Med J (Clin Res Ed), 1983 July 2; 287(6384); 39-43.
10.Furuta S, Akagawa N, Kamada E, Hiyama A, Kawabata Y, Kowata N, Inaba A, Matthews A, Hall M, Kurimoto T. Involvement of CYP2C9 and UGT2B7 in the metabolism of zaltoprofen, a nonsteroidal anti-inflammatory drug, and its lack of clinically significant CYP inhibition potential, Br. J. Clin. Pharmacol. 2002; 54:295–303.
11.Sajani P.L., development of new analytical methods for the estimation of zaltoprofen a new nsaids in bulk drug and pharmaceutical formulations, H.K.E.S’s matoshree taradevi rampure institute of pharmaceutical sciences, Gulbarga (Karnataka), 2011-2012, 4.
12.Tatsuro Kohno, Zaltoprofen inhibits Bradykinin-mediated enhancement of glutamate receptor activity in substantia gelatinosa neurons, August 2011, Volume 113, 412.
13.Japanese pharmacopoeia, the ministry of health, labour, and welfare, zaltoprofen monograph, 15th edition, 1242-1243.
14.file:///C:/Users/admin/Downloads/Desktop/work/zalto/article/1.htm.
15.Komiya Akira et. al., Oral analgesia by non-steroidal anti-inflammatory drug zaltoprofen to manage cystoscopy-related pain: A prospective study, International Journal of Urology, 2009, 874–879.
16.Tang HB, Inoue A, Oshita K, Hirate K, Nakata Y. Zaltoprofen inhibits bradykinin-induced responses by blocking the activation of second messenger signalling cascades in rat dorsal root ganglion cells. Neuropharmacology 2005; 48: 1035–42.
17.Hirate K, Uchida A, Ogawa Y, Arai T, Yoda K. Zaltoprofen, a non-steroidal anti-inflammatory drug, inhibits bradykinin-induced pain responses without blocking bradykinin receptors. Neurosci. Res. 2006; 54: 288–94.
18.Muratani T, Doi Y, Nishimura W, Nishizawa M, Minami T, Ito S. Preemptive analgesia by zaltoprofen that inhibits bradykinin action and cyclooxygenase in a post-operative pain model. Neurosci. Res. 2005; 51: 427–33.
19.Lee HW, Seo JH, Kim YW, Jeong SY, Lee KT. Determination of zaltoprofen in human plasma by liquid chromatography with electrospray tandem mass spectrometry: application to a pharmacokinetic study. Rapid Communications in Mass Spectrometry. 2006; 20(18):2675-2680.
20.Natsuko kusunoki et al, Mod Rheumatology (2008), sep.18: 542-551.
21.Krishna EH, Gupta VRM and Jyoti S.: Spherical crystallization of zaltoprofen for enhancement of micromeritic properties and dissolution rate. Int J Pharm Sci Res, 2012; Vol. 3(7): 2024-2030.
22.E. Hari Kishan, directly
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









