{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Narinder Singh*, Surya Prakash Gautam, NeelamKumari,Rupinder Kaur,Manpreetkaur
CT Institute of Pharmaceutical Sciences, Shahpur, Jalandhar, Punjab
*pharmacist.narinder@gmail.com
ABSTRACT
Virosomes are reconstituted viral envelopes that can fill in as vaccines and as vehicles for cell conveyance of different macromolecules. The prospect of drug delivery and targeting systems utilizing virosomes is an intriguing innovative work field. Since virosomes are biocompatible, biodegradable, non-poisonous and non-autoimmunogenuic; endeavors have been made to use them as antibodies or adjuvants and also conveyance frameworks for drugs and organic for remedial purposes. The achievement of virosomal medicate conveyance relies on upon strategy used to set up the typified bioactive materials and fuse them into the virosomes. Virosome innovation could conceivably be utilized to convey peptides, nucleic acids or, then again qualities and medications like anti-toxins, anticancer agents, and steroids.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2538
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 9 Received On: 30/06/2017; Accepted On: 20/07/2017; Published On: 01/09/2017 How to cite this article: Singh N, Gautam SP, Kumari N, Kaur R, Kaur M;Virosomes as Novel drug delivery System: An Overview; PharmaTutor; 2017; 5(9);47-55 |
INTRODUCTION
The Novel invention of therapeutics against neurodegenerative or cancer disorders involve delivery systems to facilitate target drugs toward particularhost tissues and cell types by controlled release and receptor-mediated uptake. Virosomal technology present anovel difficult delivery system towardconvenethese confront. Toward enhance the effectiveness of gene delivery through the beginning of molecules directly into cells.Virosomes has been developed in combining various agents like antigen, drug and DNA amongfusiongenic viral cover proteins.[1]
The Enhancement of delivery competence in vivo is a major objective in the field of virosomes research. In spite of improvements in non-viral and viral vector systems, major hurdle in the delivery of drugs and other macromolecules into the preferred cell category is crossing the permeability barrier imposed by the plasma membrane followed by the controlled release inside the cytoplasm. Virosomes are regenerateempty influenza virus envelopes. Wherever infectious neuclocapside be replaced by compound of choice. Virosomes are not replicate but they are pure fusion activity vesicles thus deliver the incorporated compound such as drug, antigen, genes inside the target cell. Virosomes protect pharmaceutically active substances from proteolytic degradation and low pH within endosomes, allowing their contents to remain intact when they reach the cytoplasm.[5]The virosome carrier systemin excess ofnewly drugdeliveryvehicles like proteoliposomal and liposomal carrier systems.
Virosome Structure
Virosomes are consist of spherical or unilamellar phospholipidbilayervesicle having meandiameter in the range of 120-180nm. Influenza virus is most commonly used for virosome production and genetic material of the sourcevirus. Virosomesarenotcap able to replicate other thanpurefusion active vesicles are presents.
Structure of Virosome
The Virosomemainly constituentsof Immune stimulating Regenerate Influenza Virosomes (IRIVs) consist of naturally occurring phosphatidylcholine (PC) and phospholipids(PL). PC formsaround 70% of the virosomal structure. The left behind 30%of membrane components has the envelopephospholipids originating from the influenza virus to facilitate provide haemagglutinin(HA) and neuraminidase (NA) glycoproteins.Virosomes canbe optimized used for maximal inclusion of thedrug or for the greatest physiological effect bymodifying the content or else type of membranelipids used. It is even possible to generatecarriers for antisense-oligonucleotides orothersome genetic molecules depending onwhether positively or negatively loadedphospholipids are included into themembrane. Various ligandslike peptides, cytokines, and monoclonalantibodies (MAbs) can be incorporated intothe virosome. It also displayed on the virosomalsurface. Tumor-specific monoclonalantibody fragments (Fab) might be linked tovirosomes to direct the carrier to selectedtumor cells.
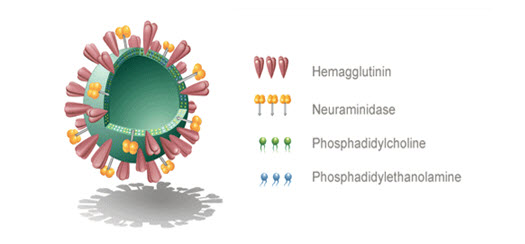
Figure no.1: Structure of Virosome
Preparation of Virosome
1. Selection of virus
Virosome are recons titute dviralenvelop which have consequent from different virus. Influenza virus envelope is most of ten used to produce virosome but virosome can also be made from Sendai virus, sindbis, Epstein-burrvirus, friendmurine leukemia virus, herpes simplex virus.[2]
2. Selection ofantigen
Antigen is preferred as per our necessities.Antigenswhich are used like abacterium parasite, carcinogenic cell, orwholecell is used as antigens. Cell components includeRNA, DNA, or plasmid could also be used as antigen.[3]
3. Reconstituted of virosome
Virosome solubilized with detergent such as (octaglucoside, nonidert p-40). Due to solubilization with detergent genetic material and internal viral protein will sediment afterward detergent is removed by different method like hydrophobic resins and dialysis from supernatant. By uses ultracentrifugation development viral matrix protein and nuclei capsid is removed. Antigen which has coupled to lipid anchor is mixed with surfactant or polymersolution. This solution is processamong virosome mover antigen bound virosome is achieved.[4]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Advantages of virosomal drug delivery [6]
• Enable medication conveyance into the cytoplasm of target cell
• Virosomes are biodegradable.
• Protects drugs against corruption.
• Biocompatible, and non-lethal
• No ailment transmission hazard
• No autoimmunogenity
• Broadly material with terrifically imperative medications (anticancer drugs, proteins, peptides, nucleic acids, anti-infection agents, fungicides)
• Promotes combination movement in the endolysosomal pathway
• Target-specificdeliveryofantigensandamplification of the immune response.
• Extended uptake, distribution andeliminationofdruginbody.
• Up scaling according to standard procedure.
• Virosomeallowpatientspecific modular vaccine regimen.
• Can be regulated by injection or nasally.
• Release of active substance in cytosol of selected cell. The anti genis partially protect ed from extra cellular degradation and theres ulting depot effect greatly facilitates immune potentiation.
• Target-specificdeliveryofantigensandamplificationoftheimmuneresponse.
• Virosomeallow tolerant specificmodular vaccine regimen.
Characterization of virosomes
Protein detection
Virosome preparation could be usually consequence in the comparatively consistent protein to lipid ratios. For the confirm the presence of HA protein in the virosomes the agent Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) are used.[7]
Structure and size
For the Negative stain electron microscopy should be normally be used to determine the ultrastructure and size of virosomes. The Prepared staining solutions could be rather be of neutral pH for avoid acid induced conformation alteration of HA.[8]
Fusion activity
Regularly virosomes show pH dependent film combination movement like local flu infection. Virosomal combination with organic or counterfeit target films can be surveyed in vitro with an excimer measure utilizing pyrene-named lipids, where the decline of surface thickness of the pyrene-phosphatidyl choline-name on combination with an unlabeled layer compares to a decrease of excimer fluorescence. Combination action additionally can be in a roundabout way checked by deciding hemolytic action, which relates nearly to combination action and displays pH reliance indistinguishable with that of combination.
Evaluation of Virosomes[9].
A) Surface morphology and vesicle shape: - Transmission electron microscopy, Freeze break electron microscopy.
B) Size dispersion and vesicle size:- Dynamic light scrambling, Transmission electron microscopy, Zetasizer, Photon connection spectroscopy, Laser light diffusing, Gel saturation and gel avoidance.
C) Surface charge: - Free stream electrophoresis.
D) Surface pH and electrical surface potential: - Zeta potential estimations and pH touchy tests.
E) Lamellarity: - Small edge x-beam dissipating, Freeze break electron microscopy, 13p-NMR.
F) Phase conduct: Freeze crack electron microscopy, Differential checking colorimetry.
G) Percent of free medication: Mini section centrifugation, Gel avoidance and Ion trade chromatography, Protamine accumulation, Radiolabelling.
H) Drug discharge: Diffusion cell/dialysis.
I) Pyrogenicity: Rabbit fever reaction test or Limulus ambeocyte lysate (LAL) test.
J) Animal poisonous quality: observing survival rates, histology and pathology.
K) Chemical examination of surface: Static auxiliary particle mass spectrometry.
Applications of Virosome Technology
Viruses are commit intracellular parasites as they are fundamentally subordinate upon particular host cells for their survival. This guideline leads to the improvement of a medication conveyance framework that copies the viral example of cell disease. Virosomes are made out of a phospholipid bilayer with the viral surface glycoproteins distending from the surface of these vesicles. The synthesis of the vesicular layer empowers the virosomes to be biocompatible and biodegradable. They are productively consumed and circulated to the objective site without being modified by the physiological procedures of the body. Additionally, the plan and structure of virosomes is to such an extent that medication particles of various nature can be consolidated in them. The lipid bilayer can effortlessly incorporate the hydrophobic medications in it. Hydrophilic medications, on the other hand, turn into a piece of the focal lacunae[10-15].Virosomes can be coupled to an immune response to guarantee the focused on conveyance of a helpful operator to upgrade the tissue specificity. These antibodies tie to the particular receptors of cells supporting the conveyance of medication atoms to these objectives. This property can, particularly, be used for conveying the medication particles with limit security profiles. Disease chemotherapeutic operators, for example, can be conveyed particularly to the tumors by marking the virosomes with antibodies [16-17]. Virosomes have appeared to viably transport macromolecules including drugs, nucleic acids and proteins to different cell sorts including hepatocytes, erythrocytes, safe cells and gliomacells.Variousvirosome based items have been affirmed by the United States Food and Drug Administration (FDA) for human utilize .The surface glycoproteins of Influenza infection, hepatitis infections and vesicular stomatitis infection have been effectively consolidated in various antibody and medication conveyance system[18-22]. Virosomes containing malignancy chemotherapeutic specialists, antimalarial, antibacterial and antifungal operators have appeared effective discharge profiles in vitro and in vivo. In view of the same standard, bacterial apparitions have been created. These vesicles contain the external shell or the encompass protein of different gram negative microscopic organisms [23-26]. These bacterial apparitions emulate a comparative design as is seen in the event of a characteristic disease. The virosome based sedate conveyance is, in any case, quick, sheltered and viable rather than other related system.
Virosomes as Immunopotentiating Agents
Virosomes are the agents that can serve the function of delivering antigens and drugs to specific cell types. The chief property exploited throughthe virosome design is the interaction amongst the antigenic proteins of the virus with the cellular receptors [38]. Moreover, the identification, uptake and representation of the antigen incorporated in the virosomeby the relevant antigen presenting cells helps in stimulating the immunesystem. As a result efficient regulatory and effector immune responses aregenerated. They initiate both cell mediated and humor alarms of immune system. Additionally, virosomes induce bothcytotoxic and helper T-cell responses [27-29].Virosomes cannot only serve as a means to transfer the immunogen to the body but can also act as adjuvants for directing the immune response to the particular antigen. They, being of particulate nature, can easily attract the dendritic cells and other antigen presenting cells for attaining immunological benefits. The composition of thevirosome ensures that the antigen, whether intercalated into the lipid bilayer, conjugated to the surface proteins or present in the central cavity, is delivered continuously in a sustained manner to the immunesystem [30-31]. This delay in the release of the antigen can act as a tool for focusing the immune response to the particular antigen in order to gain a depot-like effect. Furthermore, the combined delivery of the antigen and the adjuvant can help in the attainment of an exaggerated immune protection against various diseases. Recent studies on murine models have exhibited up to four-fold improved humoral response in case of virosome based product in comparison to that observed on the delivery of nascent antigen [32-33].
Virosomes as Agents of Targeted Drug Delivery
One of the imperative essentials of a medication conveyance framework is to transport a remedial specialist successfully to the objective site in an auspicious way. Keeping in mind the end goal to help the focused on sedate conveyance, drugs should be either changed or bundled in such a way, to the point that restoratively successful amounts of medication atoms achieve the site of activity. Change might include the modification of physical as well as synthetic parameters of the medication bringing about the generation of new synthetic elements, blending with other substance constituents to alter their in vivo discharge profiles or, on the other hand change of physical structures of the medication particles.Virosomes can bundle medications of an assortment of nature in themselves [33-34]. They can fill in as fantastic intends to convey hydrophilic and hydrophobic medication atoms to a particular kind of tissue. The water-cherishing or hydrophilic medications are epitomized in the focal compartment amid the virosome generation prepare. The lipophilic drugs, then again, can't be epitomized in this way and are, along these lines, installed in the lipid bilayer. The moderate breaking down what's more, disintegration of the virosomes inside the cell can fill in as a methods of conveying these medication particles to the expected site of activity. The exemplification of different types of hereditary material in the virosome, to be utilized for prophylactic or helpful purposes, has been accomplished in various examinations. The lipid bilayer of the virosome makes a difference in the assurance of these remedial operators from different nucleic corrosive corrupting proteins including DNAases and RNAases. The viral glycoproteins subsequent to perceiving the particular cell sorts help in the combination of the films. The hereditary material once conveyed can, at that point, be used by the cell hardware for the creation of the encoded qualities [35-36].
Virosome-Cell Interaction
The main preferred standpoint of the virosome innovation is their ability to mimic an in vivo contamination express that can be useful in drawing in the resistant players and the arrangement of macromolecules to the separate site of activity. Virosomes perceive and tie to the same receptors that are used if there should be an occurrence of a characteristic viral disease. Sialic corrosive receptors, for example, are used by the flu virosomes. After the cell receptor acknowledgment by the infection, combination of viral and endosomal layer is watched. If there should arise an occurrence of flu virosomes, for instance, the hemagglutinin (HA) viral protein uses its dipartite get together for a similar reason [37-38]. Furthermore, the neuraminidase (NA) is likewise incorporated into the virosome get together as it can upgrade the immunogenicity and focusing of the virosome to a specific tissue. The virosome-receptor connection has been explored for the treatment of various sicknesses including parasitic illnesses, viral maladies, neurological clutters and numerous other metabolic issue [39]. In every one of the cases, the principle point is the arrangement of a nano-sized protein, nucleic corrosive or a medication particle to the expected site of activity. Peptides and proteins have been effectively conjugated with the virosome-surface glycoproteins. Immunizations have been produced against the Respiratory Syncytial Virus (RSV) utilizing the flu virosomes by intertwining the monitored proteins of the Hepatitis C infection surface proteins with the virosomal proteins positive enlistment of cytotoxic White blood cell insusceptible reaction. Correspondingly, epitopic locales of B-cell have been conceived utilizing flu virosomes particularly against intestinal sickness [40]. Moreover, numerous pathogens have been focused on utilizing the virosome framework. This system, along these lines, helps in maintaining a strategic distance from rehashed and different dosing for vaccination purposes.
Pharmacokinetics of Virosomes
Pharmacokinetics data can be utilized to translate the distinctions in the pharmacological impact of liposomal-entangled medication and free medication, consequently can be abused for measurements planning. Pharmacokinetics manages time course of retention, dissemination and debasement of the virosomal transporters in vivo. The pharmacokinetics of virosomes requires the information of conceivable available locales after intravenous organization as this is the most acknowledged course for different virosomal details misused for clinical therapeutics with the exception of topical plans. Virosomes modifies both the tissue dissemination and the rate of leeway of a medication as they are influenced by the pharmacokinetics parameters. Under ideal conditions the medication has been conveyed inside the virosomal fluid stage amid flow and it spills at adequate rate to wind up noticeably bioavailable on landing in tissue or other particular destinations. Bioavailability in the event of virosomal transporters can be characterized as the measure of free medication that can get away from the bounds of the bearer and in this manner end up plainly accessible for redistribution to neighboring tissue[41].
Mechanism of Action of Virosomes
Virosomes act both as a medication conveyance transporter and furthermore as an adjuvant with different capacities amid the enlistment of a safe reaction to body's. The transporter work contains the beneficial outcomes of implanting the antigen into a higher structure, the virosome molecule. The adjuvant capacity identifies with immune stimulating properties of the virosomes and their segments on the resistant framework. Above all, virosomes prevail with regards to invigorating particular invulnerability without causing nonspecific aggravation [42].
Carrier Function Response
The mix of the antigen into the higher structure of the virosome molecule balances out the antigen to jam the local status of B cell epitopes and shields the antigen from debasement. The antigen shown at first glance virosomals which mirrors the first pathogen or target cell and along these lines supports the era of antibodies important for security. The introduction of the antigen as a tedious surface structure upgrades its acknowledgment by immunizer creating B-cells. The size and surface structure of the virosome particles make them an alluring focus for take-up and handling by safe cells and which is a vital stride in the start of an invulnerable reaction [43].
Virosomes Adjuvant Function
The adjuvant capacity of virosomes depends on the nearness of flu determined envelope proteins, specifically the overwhelming haemagglutinin (HA). Prior antibodies against flu tie to virosomes and label them productively for fast take-up and preparing by antigen displaying cells (APC). The common capacity of antibodies is to tie infection and piece disease. Previous flu particular partner T cells are enacted by those antigen exhibiting cells (APC) showing the prepared sections of the flu proteins. Flu proteins enacted aide cells quickly multiply and discharge cytokines to help and improve the enlistment of impact or safe cells, e.g. immune response creating cells [44].
Difference between Virosomes and Liposomes
Viral envelope glycoproteins with receptor-official and layer combination properties that empower the phone liposomes have been viewed as promising vehicles for focusing on and conveyance of organically dynamic atoms to living cells both in vitro and in vivo. Liposomes can possibly combine with cells and by and large neglect to give obvious conveyance of epitomized atoms to the cell cytoplasm. Virosomes containing practical conveyance of epitomized particle[45].
Virosome Uptake by Cells
a. Attachment
This includes official of the virosomes by means of HA to the cell receptors that are a film glycoprotein or glycolipid with terminal sialic corrosive. If there should be an occurrence of particular virosomes, Fab sections are coupled by a cross-linker with a spacer arm to the virosomal surface. Particular virosomes will also perceive antigenic structures on the focusing on cell surface and bringing about a connection to target cells by two distinctive restricting components. Along these lines, particular virosomes apply selectivity for extraordinary cell sorts.
b. Penetration
After Penetration entry of virosomes happens by receptor-intervened endocytosis. The virosomes are caught in endosomes, acidic combination of the virosomal film with endosomal layer. The combination is interceded by the viral spike glycoprotein hemagglutinin (HA). The layer combination response in the endosome librates the virosomes from its lipid envelope and gives access to the embodied medications to the cytosol.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Functions by the Carrier
The reconciliation of the antigen into the higher structures of the virosomes molecule balance out the antigens, safeguards the local status of B cell epitopes and shields the antigens from debasement. In addition, the introduction of the antigen as a monotonous surface structure improves its acknowledgment by counter acting agent creating B cells.
Memory Support
The nearness of flu inferred hemagglutinin (HA) incites a memory reaction as a greater part of individuals have a level of common, prior insusceptibility against flu. This contains both humeral and cell invulnerability: previous flu particular antibodies tag virosomes proficiently for fast take-up and handling by antigen introducing cells (APC).
Memory T partner cells rapidly multiply besides discharge cytokines to help and upgrades Target-specific delivery of antigens and amplification of the immune response.
• Extended uptake, distribution and elimination of drug in bodythe enlistment of effector resistant cells.
Table no.1: Current Study of formulation and recovery
|
Molecules |
Development or Research Indications |
Refrences |
|
Mumps F/HN DNA plasmid |
Research |
[46] |
|
PTH-rP DNA plasmid |
Research |
[47] |
|
CEA and CD40L DNA plasmid |
Research |
[48] |
|
Antisense L-myc OPT |
Research |
[49] |
|
F RSV protein |
Research |
[50] |
|
Fab' Her-2 Neu and doxorubicin |
Research |
[51] |
|
mAb anti epithelial glycoprotein 2 |
Research |
[52] |
|
Plasmodium falciparum SPf66 peptide |
Research |
[53] |
|
Plasmodium falciparum AMA-1 peptide |
Phase I clinical trial (PEVION) |
[54] |
|
HCV peptides |
Research |
[55] |
|
Amyloid Ab peptides |
In development (PEVION) |
[56] |
Table no.2: Virosome Based Products Approved and under review by regulatory authorities.
|
Therapeutic/Prophylactic Purpose |
References |
|
Influenza virus vaccine |
[57] |
|
Hepatitis A virus vaccine |
[58] |
|
Hepatitis B virus vaccine |
[59] |
|
Hepatitis C virus vaccine |
[60] |
|
Antifungal agents |
[61] |
|
Cancer chemotherapy |
[62] |
|
Antiparasitic agents |
[63] |
CONCLUSION
Virosomes can be used to deliver an antigen in the host body throughalteredroutes like intranasal, intradermal, and intramuscular, depending on the aim of immunization deprived of side effects. Virosomes could also be abused as bearers for targeted drugs and for immunomodulating molecules particularly in cancer treatment. An attractive feature of virosomes is the possibility to target them to chose cells utilizing Fab pieces of monoclonal antibodies particular for the binding. Influenza virosomes can be an abundant device for delivering antigens and atoms of various nature, for example, proteins, peptides, plasmids, oligonucleotides and even medications, to cells and because of their flexibility and it is conceivable to abuse them in various logical divisions. .
REFERENNCES
1. Targeting HER-2/neu with AntiratNeu Virosomes for Cancer Therapy, Cancer Res ;62;437-444
2. Stegmann T., Morselt HWM., Booy FP., Van Breemen JFL., Scherphof G., Wilschut J; Functional reconstitution of influenza virus envelopes; EMBO. J; 1987; 6;2651–9
3. Bron R., Ortiz A., Wilschut J; Cellular cytoplasmic delivery of a polypeptide toxin by reconstituted influenza-virus envelopes (virosomes); Biochem;1994; 33;9110–17
4. Schoen P., Bron R., Wilschut J Delivery of foreign substances to cells mediated by fusion-active reconstituted influenza virus envelopes (virosomes). J Liposome Res. 1993; 3:767–92.
5. ZinkernagelRM,HengartnerH;Regulationoftheimmuneresponsebyantigen.Science;2001;293;251-3
6. MetsikköK,vanMeerG,SimonsK.Reconstitutionofthefusogenicactivityofvesicularstomatitisvirus.EMBOJ;1986;5;3429–35
7. Huckriede A., Bungener L., Stegmann T., Daemen T., Medema J., Palache AM; The virosome concept for influenza vaccines;Vaccine; 2005; 23;26–38
8. Felnerova D., Viret JF., Glück R., Moser C; Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs; CurrOpinBiotechnol;2004; 15;518–29
9. Bhamare G.,Karpe M., Kadam V;virosome drug and vaccine delivery system;wjpps;2014;3(10);437-447
10. Kaneda Y;Virosomes: evolution of the liposome as a targeted drug delivery system; Adv Drug Deliv Rev;2000; 43;197–205
11. Moser C., Amacker M; Influenza Virosomes as Antigen Delivery System. In: Novel Immune Potentiators and Delivery Technologies for Next Generation Vaccines; Springer US; 2013; 287-307.
12. Koppers-Lalic D., Hogenboom MM., Middeldorp JM., Pegtel DM; Virus modified exosomes for targeted RNA delivery; a new approach in nanomedicine ; Adv Drug Deliv Rev ;201365: 348-356.
13. Moser C., Amacker M., Zurbriggen R; Influenza virosomes as a vaccine adjuvant and carrier system; Expert Rev Vaccines;2011; 10; 437-446
14. Radha GV., Rani TS., Sarvani B; A review on proniosomal drug delivery system for targeted drug action. Journal of Basic and Clinical Pharmacy;2013;4;42-48
15. Soussan E., Cassel S., Blanzat M., Rico-Lattes I;Drug delivery by softmatter: matrix and vesicular carriers;AngewChemInt Ed Engl;2011;48; 274-288
16. Bhattacharya S, Mazumder B; Virosomes: A Novel Strategy for Drug Delivery and Targeting; Bio Pharm International; 2011; 24: 9-14.
17. Huckriede A., Bungener L., ter Veer W., Holtrop M., Daemen T., et al;Influenzavirosomes: combining optimal presentation of hemagglutinin with immune potentiating activity. Vaccine;2003;21;925-931
18. Goyal AK., Khatri K., Mishra N., Vyas SP ;New patents on mucosal delivery of vaccines. Expert Opinion on Therapeutic Patents;2008;8;1271-1288
19. Nordly P., Madsen HB., Nielsen HM., FogedC;Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immune stimulators. Expert Opin Drug Deliv ;2009;6; 657-672
20. Hatz C., van der Ploeg R., Beck BR., Frösner G., Hunt M, et al; Successful memory response following a booster dose with a virosome-formulated hepatitis a vaccine delayed up to 11 years. Clin Vaccine Immunol; 2011;18; 885-887.
21. Miyanohara A; Preparation of vesicular stomatitis virus-G (VSV-G)conjugate and its use in gene transfer. Cold Spring Harb Protocol;2012;453-456
22. Torresi J., Johnson D., Wedemeyer H; Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J Hepatol; 54: 1273-1285.
23. Krishnamachari Y., Geary SM., Lemke CD., Salem AK; Nanoparticle delivery systems in cancer vaccines. Pharm Res; 2011; 28;215-236.
24. Weeratna RD., McCluskie MJ; (2011) Recent Advances in Vaccine Adjuvants; Emerging Trends in Antibacterial Discovery: Answering the Call to Arms;2011;303-320.
25. Muhammad A., Champeimont J., Mayr UB., Lubitz W., Kudela P ; Bacterial ghosts as carriers of protein subunit and DNA-encoded antigens for vaccine applications. Expert Rev Vaccines;2012;11; 97-116
26. Kudela P., Koller VJ., LubitzW;Bacterial ghosts (BGs)--advanced antigen and drug delivery system;Vaccine ;2011;28; 5760-5767
27. Henriksen-Lacey M., Korsholm KS., Andersen P., Perrie Y., Christensen D; Liposomal vaccine delivery systems; Expert Opin Drug Deliv;2011;8: 505-519.
28. Wilschut J; Influenza vaccines: the virosome concept. ImmunolLett ;2009;122;118-121.
29. Joshi MD., Unger WJ., Storm G., van Kooyk Y., Mastrobattista E; targeting tumor antigens to dendritic cells using particulate carriers. J Control Release;2012;161; 25-37
30. Alving CR., Peachman KK., Rao M., Reed SG; Adjuvants for human vaccines. CurrOpin Immunol;2012; 24; 310-315
31. Reed SG., Bertholet S., Coler RN., Friede M ; New horizons in adjuvants for vaccine development. Trends Immunol;2009; 30; 23-32
32. O’Hagen DT., Wack A; Adjuvants: From Serendipity to Rational Discovery. Volcanology: Principles and Practice;2012; 348.
33. Zurbriggen R., Novak-Hofer I., Seelig A., Glück R;IRIV-adjuvant hepatitis A vaccine: in vivo absorption and biophysical characterization; Prog Lipid Res ;2000;39; 3-18
34. WilschutJ;Influenza vaccines: the virosome concept; Immunol Lett;2009; 122;118-121
35. Chen DL., Zheng D., Paller AS; Nano-Based Gene Therapy forDermatologic Diseases. In: Nanotechnology in Dermatology; Springer NewYork; 109-117.
36. Liu J., Wu J., Wang B., Zeng S., Qi F., et al; Oral vaccination with aliposome-encapsulated influenza DNA vaccine protects mice against respiratory challenge infection. J Med Virol.Torchilin V Membrane Barriers for Bringing Drugs inside Cells and Inside Cell Organelles; J MembraSciTechnol; 2013;114.
37. Liu H., de Vries-Idema J., Ter Veer W., Wilschut J., Huckriede A; Influenza virosomes supplemented with GPI-0100 adjuvant: a potent vaccine formulation for antigen dose sparing; Med Microbiol Immunol;2013
38. Markovic I., Leikina E., Zhukovsky M., Zimmerberg J., ChernomordikLV;Synchronized activation and refolding of influenza hemagglutinin in multimericfusion machines; J Cell Biol;2001; 155; 833-844
39. Pastori C., Wahlestedt C; involvement of long noncoding RNAs in diseases affecting the central nervous system; RNA Biol;2012; 9; 860-870
40. NardinE;The past decade in malaria synthetic peptide vaccine clinical trials; Hum Vaccin;2010; 6; 27-38
41. Rajat S., MohdYasir; Virosomes: A Novel Carrier for Drug Delivery; International Journal of Pharm Tech Research;2010;2(4); 2327-2339
42. 29. Huckriede A., Bungener L., ter Veer, W; Influenza virosomes: combining optimal presentation of hemagglutinin with immuno potentiating activity;Immunopotentiators in Modern Vaccines;2003;21(9-10); 925–931
43. Huckriede A., Bungene, L., Stegmann, T;Thevirosome concept for influenza vaccines;2005; 23(1);26–38
44. Kaneda Y; Virosomes: evolution of the liposome as a targeted drug delivery system;Adv Drug Deliv Rev;2000; 43(2-3); 197–205
45. Stegmann T., Morselt H.W., Booy, FP; Functional reconstitution of influenza virus envelopes; EMBO J;1987; 6(9): 2651-2659
46. Waelti E., Wegmann, N., Schwaninger, R; Targeting HER/neu with antiratNeuvirosomes for cancer therapy; Cancer Res;2002;62(2); 437-444
47. jonge J., Holtrop M., WilschutJ;Reconstituted influenza virus envelopes as an efficient carrier system for cellular delivery of small-interfering RNAs;Gene Ther;2005;13(5); 400-411
48. Markus., SM., Annabelle, R., Francesca, B;. Induction of parasite growth inhibitory antibodies by a virosomal formulation of a peptidomimetic of loop I from domain III of Plasmodium falciparum apical membrane antigen1;Infec Immun;2003; 71(8);4749-4758
49. Maria GC., Rinaldo Z., Marcello V;Intranasal immunization with mumps virus DNA vaccine delivered by influenza virosomes elicits mucosal and systemic immunity; Virology ;2000;277(1); 111-118
50. Cusi MG., Del Vecchio., M.T, Terrosi C; Immune-Reconstituted Influenza Virosome containing CD40L gene enhances the immunological and protective activity of a carcinoembryonic antigen anti-cancer vaccine. J Immunol ;2005;174(11); 7210-16
51. Dijkstra J., Bron R., Wilschut J; Activation of murine lymphocytes by lipopolysaccharide incorporated in fusogenic, reconstituted influenza virus envelopes (virosomes); J Immunol;1996; 157(3); 1028- 1036
52. Waelti ER., Glück R; Delivery to cancer cells of antisense L-myc oligonucleotides incorporated in fusogenic, cationic-lipid-reconstituted influenza-virus envelopes (cationic virosomes);Int J Cancer;1998; 77(5); 728-733
53. Pöltl-Frank F., Zurbriggen R., Helg A; Use of reconstituted influenza virus virosomes as an immunopotentiating delivery system for a peptide-based vaccine; ClinExpImmunol ;1999;17(3): 496- 503
54. Scardino A., Correale P., Firat H; In vivo study of the GC/90IRIV vaccine for immune response and autoimmunity into a novel humanised transgenic mouse; Br J Cancer;2003;89(1); 199-205
55. CusiMG.,Zurbriggen R., Correale,P;. Influenza virosomes are an efficient delivery system for respiratory syncytial virus-F antigen inducing humoral and cell-mediated immunity.Vaccine;2002; 20(29-30); 3436-3442.
56. Enrico M., Pieter S., Jan W; targeting influenza virosomes to ovarian carcinoma cells; FEBS Letters; 2001; 509(1); 71-76.
57. Mischler R., Metcalfe IC;Inflexal V a trivalent virosome subunit influenza vaccine: production;2002; 20;17-23
58. Hatz C., Beck B., Steffen R., Genton B., d’Acremont V; Real-life versus package insert: a post-marketing study on adverse-event rates of the virosomal hepatitis A vaccine Epaxal® in healthy travelers;2011;29; 5000-5006
59. GlückR;Adjuvant activity of immunopotentiating reconstituted influenza virosomes (IRIVs). Vaccine;1999;17; 1782-1787
60. Hunziker IP., Grabscheid B., Zurbriggen R., Glück R., Pichler WJ; In vitro studies of core peptide-bearing immunopotentiating reconstituted influenza virosomes as a non-live prototype vaccine against hepatitis C virus.Int Immunol;2002;14; 615-626
61. Roy RM., Klein BS; Dendritic cells in antifungal immunity and vaccine design; Cell Host Microbe; 2012; 11: 436-446.
62. Waelti E, Wegmann N., Schwaninger R., Wetterwald A., Wingenfeld C; Targeting her-2/neu with antiratNeuvirosomes for cancer therapy;Cancer Res;2002;62; 437-444
63. GentonB;Malaria vaccines: a toy for travelers or a tool for eradication?Expert Rev Vaccines;2008; 7; 597-611
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









