About Authors:
Manoj Kumar Jadia1*, U.L.Narayan2
1 Department of Pharmaceutical Chemistry, Indira Gandhi Institute of Pharmaceautical Sciences, I.R.C. Village, Bhubaneswar, Dist:Khurda, Odhisa -751015
2 Principal, Department of Pharmaceutical Chemistry, Indira Gandhi Institute of Pharmaceautical Sciences, I.R.C. Village,
Bhubaneswar,
Dist:Khurda, Odhisa -751015
Abstract:
Two methods are described for the simultaneous determination of Paracetamol and Chlorzoxazone in binary mixture. The first method was based on UV-spectrophotometric determination of two drugs, using simultaneous equation method. It involves absorbance measurement at 282.5 nm (λmax of Chlorzoxazone) and 248.0 nm (λmax of Paracetamol) in methanol; linearity was obtained in the range of 5 – 25 μg.mL-1 for both the drugs. The second method was based on HPLC separation of the two drugs in reverse phase mode using Promosil C18 column. Linearity was obtained in the concentration range of 100-500 μg.mL-1 for Paracetamol and 50-250 for the Chlorzoxazone. Both these methods have been successively applied to pharmaceutical formulation and were validated according to ICH guidelines.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1254
Introduction:
Paracetamol (Acetaminophen) chemically N- (4-hydroxyphenyl)-acetamide, is a widely used over-the-counter analgesic (pain reliever) and antipyretic (fever reducer)[1]. Paracetamol, is commonly used for its analgesic and antipyretic effects.[1] Its therapeutic effects are similar to salicylates, but it lacks anti-inflammatory, antiplatelet, and gastric ulcerative effects. [6] It is commonly used for the relief of headaches, other minor aches and pains, and is a major ingredient in numerous cold and flu remedies. In combination with opioid analgesics, paracetamol can also be used in the management of more severe pain such as post surgical pain and providing palliative care in advanced cancer patients. [2] The onset of analgesia is approximately 11 minutes after oral administration of paracetamol,[3] and its half-life is 1–4 hours.
It is the active metabolite of phenacetin, once popular as an analgesic and antipyretic in its own right, but unlike phenacetin and its combinations, paracetamol is not considered to be carcinogenic at therapeutic doses.[4] The words acetaminophen (used in the United States, Canada, Japan, South-Korea, Hong Kong, and Iran)[5] The chemical structure of Paracetamol is shown in Fig 1 [1]. The recommended dosage of paracetamol in adults is two 500mg tablets (i.e. 1gm paracetamol) every four to six hours, not exceeding eight tablets (4gms) in any 24 hour period .
Literature survey reveled that various analytical methods such as HPLC , GC-MS, LC-MS, HPLC-Electrospray tendem mass spectrometry and HPTLC have been reported for estimation of Paracetamol from its formulations and biological fluids.
Fig 1. Structure of Paracetamol
Chlorzoxazone chemically 5-chloro-3H-benzooxazol-2-one, Chlorzoxazone is a centrally acting muscle relaxant used to treat muscle spasm and the resulting pain or discomfort.[8] It acts on the spinal cord by depressing reflexes. It is sold as Muscol or Parafon Forte, a combination of Chlorzoxazone and acetaminophen (Paracetamol).[8] Possible side effects include dizziness, lightheadedness, malaise, nausea, vomiting, and liver dysfunction. Used with acetaminophen it has added risk of hepatoxicity, which is why the combination is not recommended.[9,10] There are very few methods reported for estimation of Chlorzoxazone in pharmaceutical dosage form, which includes a validated RP –HPLC, spectrophotometric method.
At present no HPLC and UV spectrophotometric methods are reported for the simultaneous estimation of Paracetamol and Chlorzoxazone in tablet formulation.
Therefore, it was thought worthwhile to develop simple, precise, accurate UVspectrophotometric and HPLC methods for simultaneous determination of Paracetamol and Chlorzoxazone in tablets.
[adsense:468x15:2204050025]
2. Experimental:
2.1. Materials:
Pharmaceutical grade Paracetamol and Chlorzoxazone were kindly supplied as a gift sample by Win Medicare PVT.LTD., India, used without further purification and certified to contain 99.53 % (w/w) and 99.66% (w/w), respectively on dried basis. All chemicals are of HPLC grade and were purchased from Merck Ltd. And India Rankem Laboratories, India.
2.2. UV- spectrophotometry:
A Thermospectronic model is Systronics-108 (Double beam) Spectrophotometer with 1cm. matched quartz cells was used. Standard stock solutions of 100 μg.mL-1 were prepared by dissolving 10 mg of each in 100 mL of methanol. From these stock solutions, working standard solutions having concentration 10 μg.mL-1each were prepared by appropriate dilutions. They were scanned in the wavelength range of 400−200 nm and the overlain spectrum was obtained (Fig 3). Two wavelengths 282.5 nm (λmax of Chlorzoxazone) and 248.0 nm (λmax of Paracetamol) were selected for the formation of simultaneous equation. The calibration curves were found to be linear in the concentration range of 30− 70 μg.mL-1, Paracetamol and 20-60 for Chlorzoxazone. The absorptivity coefficients of each drug at both wavelengths were determined. The concentration of two drugs in the mixture were calculated using equations [12]
CPCM= A2 ay1 – A1 ay2/ ax2 ay1 – ax1ay2 ……………… … (1)
CCHZ= A1 ax2 – A2 ax2/ ax2 ay1 – ax1ay2…………………... (2)
Where, A1 and A2 are absorbance of mixture at 282.5 nm and 248.0 nm; ax1 and ax2, absorptivities of Paracetamol at 282.5 nm and 248.0 nm, respectively; ay1 and ay2 absorptivities of Chlorzoxazone at 282.5 nm and 248.0 nm, respectively. CPCMand CCHZare concentration of Paracetamol and Chlorzoxazone in mixture. The absorptivities reported are the mean of six independent determinations (Table 1).
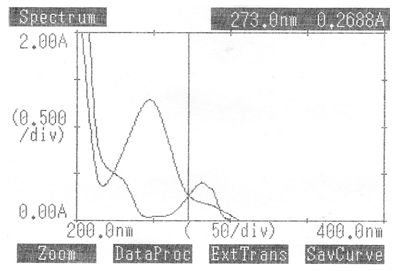
Fig 3. Overlain Spectrum of Paracetamol And Chlorzoxazone in Methanol. ATV is Paracetamol, EZM is Chlorzoxazone (each 15 μg.mL-1) taken on Systronics-108 (Double beam) Spectrophotometer
2.3. HPLC method:
The HPLC system consisted of a YL-9100 pump, a loop injector of injection capacity 20 μL, Detector consists of Photodiode array detector, a Promosil C18 (250 X 4.60 mm) 5μm column, a HPLC guard cartridge system and a YL Clarity software at ambient temperature.
Different mobile phases were tested in order to find the best conditions, for separating both
the drugs simultaneously. The optimal composition of mobile phase was determined to be TEA Buffer : Methanol in the ratio of 55:45 v/v. The flow rate was set to 1 mL.min-1 and UV detection was carried out at 240 nm. Ibuprofen was used as an internal standard.
Stock solution was prepared by dissolving 10 mg of Paracetamol and Chlorzoxazone in 100 mL volumetric flask with methanol : water in the ratio of 80:20
Table 1. Absorptivity Values at 232.5 nm (λmax of Chlorzoxazone) and 246.0 nm (λmax of Paracetamol)
|
|
Absorptivity at 226.0 nm |
Absorptivity at 256.0 nm |
||
|
|
Paracetamol |
Chlorzoxazone |
Paracetamol |
Chlorzoxazone |
|
*Mean |
342.48 |
486.87 |
467.28 |
399.86 |
|
± S.D. |
1.15 |
0.43 |
0.56 |
0.49 |
* Absorptivity values are the mean of six determinations. S.D. is standard deviation. ax1 and ax2 absorptivities of Paracetamol at 282.5 nm and 248.0 nm, respectively; ay1 and ay2 absorptivities of Chlorzoxazone at 282.5 nm and 248.0 nm, respectively.
All solutions were stored at + 50C in the dark; these solutions were shown to be stable during the period of study.
From the above stock solutions, dilutions were made in the concentration range of 100–500 μg.mL–1 of Paracetamol and 50-300Chlorzoxazone, respectivelyA volume of 20 μL of each sample was injected into column. All measurements were repeated three times for each concentration and calibration curve was constructed by plotting the peak area ratios of analyte to internal standard vs. the corresponding drug concentration.
2.4. Analysis of Pharmaceutical Dosage Forms
To determine the content of Paracetamol and Chlorzoxazone simultaneously in tablets (label claim: 10 mg Paracetamol and 10 mg Chlorzoxazone, film coated); twenty tablets were weighed; their average weight determined and were finely powdered. The correct amount of powder was dissolved in methanol by stirring for 30 min. The excipients were separated by filtration. After filtration, an appropriate amount of internal standard was added and diluted up to mark with methanol. Appropriate aliquots were subjected to above methods and the amount of Atorvastatin calcium and Chlorzoxazone were determined. The results are reported in Table 2.
Table 2. Analysis data of tablet formulations
|
Parameters |
UV – spectrophotometry |
HPLC |
||
|
Paracetamol |
Chlorzoxazone |
Paracetamol |
Chlorzoxazone |
|
|
Label Claim |
500 |
250 |
500 |
250 |
|
*Drug content |
99.95 |
98.40 |
99.22 |
99.41 |
|
± S. D. |
0.039 |
0.112 |
0.155 |
0.154 |
|
% R.S.D. |
0.127 |
0.121 |
0.159 |
0.156 |
* Value for Drug content (%) are the mean of five estimations; S.D. is standard deviation and R.S.D. is relative standard deviation
2.5. Recovery studies
To check the accuracy of the developed methods and to study the interference of formulation additives, analytical recovery experiments were carried out by standard addition method, at 80, 100 and 120 % level. From the total amount of drug found, the percentage recovery was calculated. The results are reported in Table 3.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3. Results and Discussion
Both, UV spectrophotometric and HPLC methods were found to be simple, accurate, economic and rapid for routine simultaneous estimation of Paracetamol and Chlorzoxazone, in tablet dosage forms. For UV spectrophotometric method, linearity was obtained in concentration range of 30– 70 μg .mL-1for paracetatmoland 20-60 μg .mL-1 ; with regression 0.9975 and 0.998, intercept – 0.009 and 0.004 and slope 0.0456 and 0.005 for Paracetamol and Chlorzoxazone, respectively.
Recovery was in the range of 99 – 101 %; the value of standard deviation and % R.S.D. were found to be < 2 %; shows the high precision of the method.
Table 3. Recovery studies
|
UV – spectrophotometry |
HPLC |
||||
|
Excess Drug |
*Recovery |
% R.S.D. |
Excess Drug |
*Recovery |
% R.S.D. |
|
Paracetamol |
|||||
|
80 100 120 |
99.83 99.72 99.07 |
0.2953 0.2026 0.0672 |
80 100 120 |
99.37 100.11 100.58 |
0.9405 0.0115 0.9702 |
|
Chlorzoxazone |
|||||
|
80 100 120 |
100.69 100.43 99.52 |
0.2953 0.1036 0.1165 |
80 100 120 |
100.32 99.33 98.80 |
1.1238 0.0232 1.1243 |
* Recovery is mean of three estimations.
In HPLC method, HPLC conditions were optimized to obtain, an adequate separation of eluted compounds. Initially, various mobile phase compositions were tried, to separate drugs and internal standard. Mobile phase and flow rate selection was based on peak parameters (height, tailing, theoretical plates, capacity factor), run time etc. The system with TEA Buffer : Methanol in the ratio of 55:45 v/v with 1 mL.min-1 flow rate is quite robust. A typical chromatogram for Paracetamol and Chlorzoxazone is shown in fig 4. The optimum wavelength for detection was 273 nm at which better detector response for drugs was obtained. The average retention times for Paracetamol and Chlorzoxazone was found to be 9.276± 0.03 and 5.472 ± 0.03 min, respectively. According to USP XXIV (621), system suitability tests are an integral part of chromatographic method. They are used to verify the reproducibility of the chromatographic system.
To ascertain its effectiveness, system suitability tests were carried out on freshly prepared stock solutions. The parameters obtained are shown in Table 4. The calibration was linear in concentration range of 100 – 500 μg .mL-1for paracetamol and 50-300 for chlorzoxazone with regression 0.9982 and 0.9996, intercept – 114.31 and – 305.32 and slope 50.565 and 60.58 for Paracetamol and Chlorzoxazone respectively. The low values of % R.S.D. indicate the method is precise and accurate. The mean recoveries were found in the range of 98 – 100 %.
Table 4. System suitability parameters
|
Parameters |
Paracetamol |
Chlorzoxazone |
|
Tailing factor |
1.34 |
1.21 |
|
Theoretical Plates |
6092 |
7835 |
|
Resolution factor |
2.19 |
6.08 |
|
Capacity factor |
2.50 |
5.16 |
Sample – to sample precision and accuracy were evaluated using, three samples of three different concentrations, which were prepared and analyzed on same day. Day – to day variability was assessed using three concentrations analyzed on three different days, over a period of one week.
These results show the accuracy and reproducibility of the assay. Thus, it was concluded that there was no significant difference on the assay, which was tested on an intra – day and inter – day basis.
The % R.S.D. values reported in Table 5, shows that proposed methods provides acceptable intra – day and inter – day variation of Paracetamol and Chlorzoxazone.
Ruggedness of the proposed methods was determined by analysis of aliquots from homogeneous slot in different laboratories, by different analysts, using similar operational and environmental conditions; the % R.S.D. reported in Table 5 was found to be less than 2 %.
The proposed methods are accurate, simple, rapid and selective for the simultaneous estimation of Paracetamol and Chlorzoxazone in tablet dosage form by internal standardization method. Hence, it can be conveniently adopted for the routine quality control analysis in the combination formulations. As the drug combination is available in market, hence, work is toward development of an analysis.
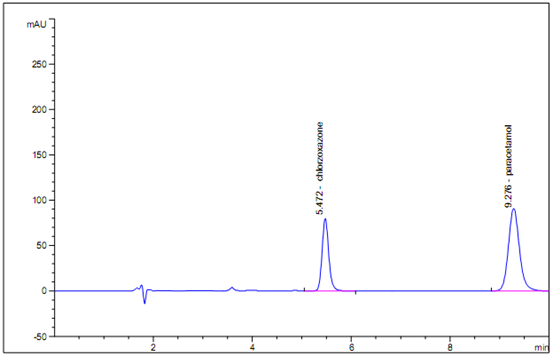
Fig 4. Chromatogram of Standard Paracetamol (100 μg.mL-1); (Rt 9.276) and Chlorzoxazone (50 μg.mL-1); (Rt 5.472) measured at 273 nm, mobile phase TEA Buffer : Methanol in the ratio of 55:45 v/v
Table 5. Summary of repeatability, precision and ruggedness
|
Parameter |
UV – spectrophotometry |
HPLC |
||
|
Paracetamol |
Chlorzoxazone |
Paracetamol |
Chlorzoxazone |
|
|
Repeatability |
1.52 |
0.09 |
0.62 |
0.57 |
|
Precision Intra-day Inter-day |
1.07 0.17 |
0.13 0.14 |
0.29 0.56 |
0.43 1.55 |
|
Ruggedness Analyst 1 Analyst 2 |
0.68 0.32 |
0.52 0.58 |
0.37 0.34 |
0.87 1.56 |
References:
1- wikipedia. org/wiki/Paracetamol/
2- Scottish Intercollegiate Guidelines Network (SIGN) (November 2008). Guideline 106: Control of pain in adults with cancer. Scotland: National Health Service (NHS). ISBN 978 1 905813 38 4
3- Moller, P.; Sindet-Pedersen, S.; Petersen, C.; Juhl, G.; Dillenschneider, A.; Skoglund, L. (2005). "Onset of acetaminophen analgesia: comparison of oral and intravenous routes after third molar surgery". British journal of anaesthesia 94 (5): 642–648. doi:10.1093/bja/aei109. PMID 1579067
4- drugbank.ca/drugs/DB00316/
5- mims.com/USA/drug/info/paracetamol/
6- jrank.org/health/pages/2881/paracetamol.html
7- pharmweb.net/pwmirror/pwy/paracetamol/pharmwebpic3.html
8- en.wikipedia.org/wiki/chlorzoxazone.
9- medicinenet.com/chlorzoxazone/article.htm
10- drugbank.ca/drugs/DB00749
11- chemicalbook.com/ProductChemicalPropertiesCB3216064_EN.htm
12- Beckett, A. H. and Stanlake, J. B., Practical Pharmaceutical Chemistry, (1997), 4 th Edition, part 2, CBS Publishers and Distributors, New Delhi (India), p 285.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









