{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Godiyal Shrikant*, Panwar Sonu, Singh Lucky
Regulatory Affairs Department, Tirupati Medicare Limited, Paonta Sahib, HP, India
ABSTRACT:
Context: To update the amendments made in Gazette Notification for changes in the Manner of Labeling of Medicine as specified in Rule 96 & 97, which shall be required to be displayed on the inner most container and every outer in which the medicines are packed on the basis of Pharmacological Category of the product under which it falls. The label of OTC medicines plays an important role in conveying valuable information to the patient for safe and effective use of OTC.
Objective: The study was conducted to evaluate the labelling of OTC drugs in India as per the guidelines of US FDA. Objective: To prepare label of the medicines as per the mandatory information to be displayed on the label of the container so that the text matter on the label is clear, readable as shall fulfill the necessary requirements of Drug & cosmetic Act.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2621
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 11 Received On: 27/09/2018; Accepted On: 05/10/2018; Published On: 01/11/2018 How to cite this article: Godiyal, S., Singh, L. and Panwar, S. 2018. Updates on the Amendments Made on the Drugs and Cosmetics Rule 1945 (Amendment Rules 2018) on Labeling of Medicines Rules 96 & 97. PharmaTutor. 6, 11 (Nov. 2018), 10-17. DOI:https://doi.org/10.29161/PT.v6.i11.2018.10. |
INTRODUCTION:
To clarify the Amendments held recently in certain Labeling Rules (2018) of Drugs and Cosmetics Rules, 1945, which was published, as required under sub-section (1) of section 12 and sub-section (1) of section 33 of the Drugs and Cosmetics Act, 1940 (23of 1940). Which are required to be printed or written under these rules, the label of inner most container of the following categories of drugs and every other covering in which the container is packed shall bear a caution or warning, as applicable, depending on whether the drug is covered under Schedule G or Schedule H or Schedule H1, Schedule X or as applicable as specified in Rule 96 & 97.
The labels of Medicines play an important role in cascading valuable information to the patients for Safe and Effective use of Medicines.
Overview of the Amendment in Manner of Labeling of Medicine Rules 96 & 97 Relating to Drugs, and Cosmetics
Rule No. 96
1. Name of the Drug (In the Drugs and Cosmetics Rules, 1945, in rule 96, in sub-rule (1), in clause (i), in sub-clause (A), for the portion beginning with the words “For this purpose” and ending with the words “name and shall be”,, the words
i. “ For this purpose, the proper name of the drug or fixed dose combination drug other than fixed dose combinations of vitamin and other fixed dose combinations containing three or more drugs shall be printed or written in a conspicuous manner which shall be in the same font but at least two font size larger than the brand name or trade name, if any and In other cases, the brand name or the trade name, if any, shall be written in brackets below or after the proper name (Ministry of Health and Family Welfare G.S.R. 222 (E).)
Explanation:
This Rule illustrate that the Brand and Generic Name of the drugs having single drug or two ingredients (fixed dose combination) will have the same font style but the Generic Name of the drugs will remain two font size larger than the Brand Name
Example in Fig.1

For the Product having fixed dose combination drug other than fixed dose combinations of vitamin and other fixed dose combinations containing three or more drugs the proper name of the drug shall be printed in the conspicuous manner and the brand name or trade name shall be written in brackets below or after the proper name.
Example in Fig.2

OR
Example in Fig.3

Earlier it was written as
96. Manner of Labelling .— (1) Subject to the other provisions of these Rules, the following particulars shall be either printed or written in indelible ink and shall appear in a conspicuous manner on the label of the innermost container of any drug and on every other covering in which the container is packed, namely :—
(i) the name of the drug−
[(A) for this purpose, the proper name of the drug shall be printed or written in a more conspicuous manner than the trade name, if any, which shall be shown immediately after or under the proper name and shall be]—( Malik Vijay)
2. In the Drugs and Cosmetics Rules, 1945 (hereinafter to be referred as the said rules), in rule 96, in sub-rule (1), in clause (xi),—
(a) for the portion beginning with the words “In addition to the’’ and ending with the words “in the above list:”, the following shall be substituted, namely:—
“In addition to the other particulars which are required to be printed or written under these rules, the label of inner most container of the following categories of drugs and every other covering in which the container is packed shall bear a caution or warning, as applicable, depending on whether the drug is covered under Schedule G or Schedule H or Schedule H1 or Schedule X, as specified in rule 97, in legible black coloured font size in a completely red rectangular box without disturbing other conditions printed on the label under these rules, namely:—
Narcotic analgesics, hypnotics, sedatives, tranquillisers, corticosteroids, hormones, hypoglycemic, antimicrobials, antiepileptics, antidepressants, anticoagulants, anti-cancer drugs and all other drugs falling under Schedules G, H, H1 and Schedule X whether covered or not in the above list:
Provided that if any of the drug referred above category is not covered under any of the Schedule, namely, Schedule G, Schedule H, Schedule H1 and Schedule X, the label of inner most container of drugs and every other covering in which the container is packed shall bear caution or warning, as the case may be, applicable for that drugs covered under Schedule H as specified in rule 97.”; (Ministry of Health and Family Welfare G.S.R. 408 (E).)
Explanation: The labeling of the innermost container and every other outer covering in which the container is packed shall be printed or written under these rules shall bear a Caution or Warning as applicable, depending on the Pharmacological category of the drugs which are covered under Schedule G, Schedule H, Schedule H1 and Schedule X as specified in Rule 97, in eligible black font size in a completely red rectangular box without interrupting other conditions printed on the label under these rules namely:
Narcotic analgesics, hypnotics, sedatives, tranquillisers, corticosteroids, hormones, hypoglycemic, antimicrobials, antiepileptics, antidepressants, anticoagulants, anti-cancer drugs and all other drugs falling under Schedules G, H, H1 and Schedule X whether covered or not in the above list:
If the drugs mentioned above are not covering under any of the Pharmacological Categories of the Product schedule, namely, Schedule G, Schedule H, Schedule H1 and Schedule X, the label of inner most container and every other outer in which the drug is packed shall bear Caution or Warning as the case may be, applicable for that drugs covered under Schedule H as specified in rule 97.
Example: For those drug which don’t comes under the preview of Schedule H warning or Caution but as per the drug and cosmetic rule 96. There are some pharmacological categories of drug like Narcotic analgesics, hypnotics, sedatives, tranquillisers, corticosteroids, hormones, hypoglycemic, antimicrobials, antiepileptics, antidepressants, anticoagulants, anti-cancer drugs which if manufactured then the label of the container shall be printed with the Warning or Caution as the case may be for that drug covered under Schedule H.
Example is mentioned below in Fig.4
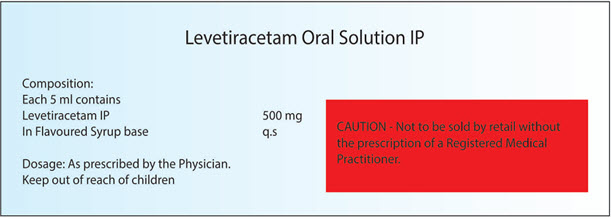
Earlier it was written as
[(xi) In addition to the other particulars which are required to be printed or written under these Rules, the label of innermost container of the following categories of drugs and every other covering in which the container is packed shall bear a conspicuous red vertical line on the left side running throughout the body of the label which should not be less than 1mm in width and without disturbing the other conditions printed on the label under these rules, namely: —
Narcotic analgestics, hypnotics, sedatives, tranquillisers, corticosteroids, hormones, hypoglycemics, antimicrobials, antiepileptics, antidepressants, anticoagulants, anti-cancer drugs and all other drugs falling under Schedules ‘G‘, ‘H‘, and ‘X‘ whether covered or not in the above list: (Garg Ram Avatar (Adv))
Rule No. 97
3. In the said rules, in rule 97, in sub-rule (1),—
(i) for clauses (a) to (e), the following clauses shall be substituted, namely:—
“(a) if it contains a drug substance specified in Schedule G, be labeled with following words in legible black coloured font size in completely red rectangular box:
(Ministry of Health and Family Welfare G.S.R. 408 (E).)
SCHEDULE G PRESCRIPTION DRUG – CAUTION
It is dangerous to take this preparation except under medical supervision’
Explanation: The drugs that come under the preview of Schedule G drugs will simply labeled with the black colour font size in completely Red Colour Rectangular Box.
Example is mentioned below in Fig.5
For Scheduled G Drug

(b) if it contains a drug substance specified in Schedule H, be labeled with symbol Rx and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black coloured font size in completely red rectangular box: (Ministry of Health and Family Welfare G.S.R. 408 (E).)
SCHEDULE H PRESCRIPTION DRUG-CAUTION
Not to be sold by retail without the prescription of a Registered Medical Practitioner.
Explanation: The drugs which are covered under Schedule H shall be labeled with symbol Rx and shall be displayed on the Left Top Corner with the below mentioned information and shall be written with eligible black color font size in completely red rectangular box. Example of such drug is given below in
Fig.6 For Scheduled H Drug (CAUTION)

(c) if it contains a drug substance specified in Schedule H and comes within the purview of the Narcotic Drugs and Psychotropic Substances Act, 1985 (61 of 1985) be labeled with symbol NRx, which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black coloured font size in completely red rectangular box:
(Ministry of Health and Family Welfare G.S.R. 408 (E).)
SCHEDULE H PRESCRIPTION DRUG-WARNING
To be sold by retail on the prescription of a Registered Medical Practitioner only.
Explanation: This Rule is applicable for only those drugs that comes under Schedule H Category but along with that also covers under the preview of the Narcotic Drugs and Psychotropic Substances Act, 1985. This shall be labeled with symbol NRx which shall be in Red Colour and displayed on the Left Top Corner with the below mentioned information and shall be written with eligible black color font size in completely red rectangular box. Example of such drug is given below in Fig.7
For Scheduled H Drug (Warning)
(under the preview of the Narcotic Drugs and Psychotropic Substances Act, 1985)

(d) if it contains a drug substance specified in Schedule X, be labeled with symbol XRx, which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black coloured font size in completely red rectangular box: (Ministry of Health and Family Welfare G.S.R. 408 (E).)
SCHEDULE X PRESCRIPTION DRUG-WARNING
To be sold by retail on the prescription of a Registered Medical Practitioner only.
Explanation: This Rule is applicable for only those drugs that come under Schedule X Category that covers under the preview of the Narcotic Drugs and Psychotropic Substances Act, 1985. This shall be labeled with symbol XRx which shall be in Red Colour and displayed on the Left Top Corner with the below mentioned information and shall be written with eligible black color font size in completely red rectangular box. Example of such drug is given below in Fig.8
For Scheduled X Drug
(under the preview of the Narcotic Drugs and Psychotropic Substances Act, 1985)

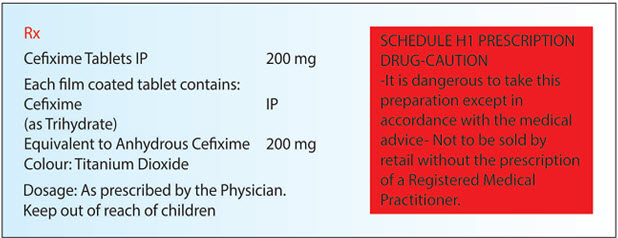
(e) if it contains a drug substance specified in Schedule H1, be labeled with symbol Rx, which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black coloured font size in completely red rectangular box: (Ministry of Health and Family Welfare G.S.R. 408 (E).)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SCHEDULE H 1 PRESCRIPTION DRUG-CAUTION
- It is dangerous to take this preparation except in accordance with the medical advice
- Not to be sold by retail without the prescription of a Registered Medical Practitioner.
Explanation: The drugs which are covered under Schedule H1 shall be labeled with symbol Rx and shall be displayed on the Left Top Corner with the below mentioned information and shall be written with eligible black color font size in completely red rectangular box. Example of such drug is given below in Fig.9
SCHEDULE H 1 PRESCRIPTION DRUG (CAUTION)
(f) if it contains a drug substance specified in Schedule H1 and comes within the purview of the Narcotic Drugs and Psychotropic Substances Act, 1985 (61 of 1985) be labeled with symbol NRx, which shall be in red and conspicuously displayed on the left top corner of the label and shall also be labeled with the following words in legible black coloured font size in completely red rectangular box: (Ministry of Health and Family Welfare G.S.R. 408 (E).)
SCHEDULE H 1 PRESCRIPTION DRUG-CAUTION
-It is dangerous to take this preparation except in accordance with the medical advice
- Not to be sold by retail without the prescription of a Registered Medical Practitioner.
Explanation: This Rule is applicable for only those drugs that comes under Schedule H1 Category but along with that also covers under the preview of the Narcotic Drugs and Psychotropic Substances Act, 1985. This shall be labeled with symbol NRx which shall be in Red Colour and displayed on the Left Top Corner with the below mentioned information and shall be written with eligible black color font size in completely red rectangular box. Example of such drug is given below in Fig.10
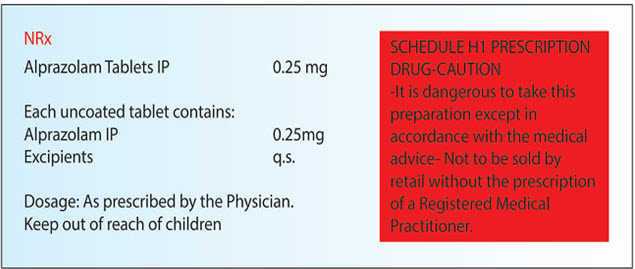
Earlier it was written as
Labelling of medicines.—
1[(1) The container of a medicine for internal use shall—
(a) if it contains a substance specified in Schedule G, be labelled with the words ‘Caution: It is dangerous to take this preparation except under medical supervision’ – conspicuously printed and surrounded by a line within which there shall be no other words;
(b) if it contains a substance specified in Schedule H, be labelled with the symbol Rx and conspicuously displayed on the left top corner of the label and be also labelled with the following words:
‘Schedule H drug- Warning: To be sold by retail on the prescription of a Registered Medical Practitioner only’.
(c) if it contains a substance specified in Schedule H, and comes within the purview of the 2[Narcotic Drugs and Psychotropic Substances Act, 1985 (61 of 1985)] be labelled with the symbol NRx which shall be in red and conspicuously displayed on the left top corner of the label, and be also labelled with the following words:
‘Schedule H drug- Warning: To be sold by retail on the prescription of a Registered Medical Practitioner only’.
(d) If it contains a substance specified in Schedule X, be labelled with the symbol XRx which shall be in red conspicuously displayed on the left top corner of the label and be also labelled with the following words :
‘Schedule X drug -Warning: To be sold by retail on the prescription of a Registered Medical Practitioner only’.
[(e) if it contains a drug substance specified in Schedule H1, the drug formulation shall be labeled with the symbol Rx which shall be in red conspicuously displayed on the left top corner of the label, and shall also be labelled with the following words in a box with a red border: (Justice Iyer V.R Krishna)
“SCHEDULE H1 DRUGS – WARNING:
- It is dangerous to take this preparation except in accordance with the medical advice.
- Not to be sold by retail without the prescription of a Registered Medical Practitioner only’.]
References:
1. Garg Ram Avatar (Adv), April 2018, Labeling and Packaging of Drugs other than Homeopathic Medicines, Manual on Drug and Cosmetics 10th Edition, Commercial Law Publisher (India) Pvt. Ltd. Delhi, Pg No. 142.
2. Justice Iyer V.R Krishna, 2015, Labeling and Packaging of Drugs other than Homeopathic Medicines, Drugs and Cosmetics Laws, Universal Law Publishing Co. Pvt. Ltd. New Delhi, Pg. No. 138,139.
3. Malik Vijay, 24th Edition 2014, Labeling and Packaging of Drugs other than Homeopathic Medicines, Law Relating To Drugs and Cosmetics 24th Edition, Eastern Book Company Lucknow, Pg No. 209,210
4. Ministry of Health and Family Welfare G.S.R. 222 (E). Drug and Cosmetics (First Amendment) Rules, 2018 published on 13.03.2018, w.e.f. 13.09.2018.
5. Ministry of Health and Family Welfare G.S.R. 408 (E). Drug and Cosmetics (Fifth Amendment) Rules, 2018 published on 26.04.2018, w.e.f. 01.11.2018.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









