About Authors:
Ipshita Chattopadhyaya, Ekta Malhotra
Department of Pharmacy,
Jaipur National University,
Jaipur, India
ABSTRACT
(1)The developments in column packing technology and suitable equipment paved the way for what is now called High Performance or High Pressure Liquid Chromatography (HPLC). The new technique provided much higher resolution, more accurate quantitative results, as well as shorter analysis times in comparison to the earlier techniques .During the sixties, new theoretical insights accompanied by important. Since its introduction, HPLC has evolved into an indispensable tool in many analytical laboratories and is applied to diverse analytical problems. Actually, HPLC refers to a number of separation techniques that use a liquid mobile phase, or eluent. Troubleshooting HPLC instrumentation and separations require a fundamental understanding of how the instrument functions and how the separation works. (2)This article provides a practical guide to common HPLC problems, along with more in-depth information to help the reader understand the relationships between the observed symptoms and the underlying causes. The practical approach presented here is meant to serve as both a troubleshooting guide and an HPLC learning tool.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1190
ABOUT HPLC
(3)Prior to the 1970's, few reliable chromatographic methods were commercially available to the laboratory scientist. During 1970's, most chemical separations were carried out using a variety of techniques including open-column chromatography, paper chromatography, and thin-layer chromatography. However, these chromatographic techniques were inadequate for quantification of compounds and resolution between similar compounds. (4)During this time, pressure liquid chromatography began to be used to decrease flow through time, thus reducing purification times of compounds being isolated by column chromatography. However, flow rates were inconsistent, and the question of whether it was better to have constant flow rate or constant pressure was debated. (5)High pressure liquid chromatography was developed in the mid-1970's and quickly improved with the development of column packing materials and the additional convenience of on-line detectors. In the late 1970's, new methods including reverse phase liquid chromatography allowed for improved separation between very similar compounds (6)By the 1980's HPLC was commonly used for the separation of chemical compounds. New techniques improved separation, identification, purification and quantification far above the previous techniques. Computers and automation added to the convenience of HPLC. Improvements in type of columns and thus reproducibility were made as such terms as micro-column, affinity columns, and Fast HPLC began to emerge. (7)The past decade has seen a vast undertaking in the development of the micro-columns, and other specialized columns. (8)The dimensions of the typical HPLC column are: 250 mm in length with an internal diameter between 3-5 mm. hope that this article will provide readers with HPLC HISTORY its operation parameters and handle trouble while operation.
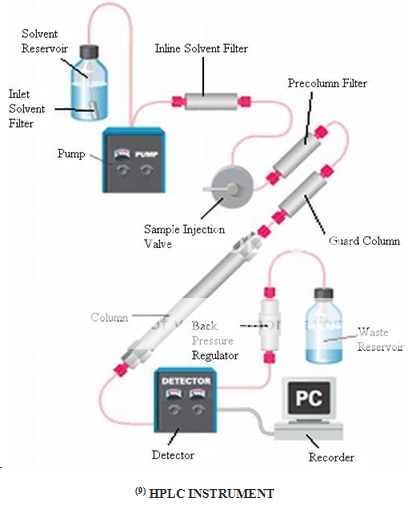
TROUBLES FACED DURING HPLC OPERATIONS AND ITS SOLUTIONS
|
Problem |
Possible cause |
Solution |
|
(10)No peaks or very small peaks |
Detector off |
Check detector |
|
Broken connections to recorder |
Check connections |
|
|
No sample/Wrong sample |
Check sample. Be sure it is not deteriorated. Check for bubbles in the vials |
|
|
Wrong settings on recorder or detector |
Check attenuation. Check gain |
|
|
No Flow |
Pump off |
Start Pump |
|
Flow interrupted |
Check reservoirs. Check position of the inlet tubing. Check loop for obstruction or air. Check degasing of mobile phase. Check compatibility of the mobile phase components. |
|
|
Leak |
Check fittings. Check pump for leaks and precipitates. Check pump seals. |
|
|
Air trapped in the system |
Disconnect column and prime pump. Flush system with 100% methanol or isopropanol. Contact servicing if necessary. |
Column and Fittings Leaks
|
Problem |
Possible cause |
Solution |
|
Column end leaks |
Loose fitting |
Tighten or replace fitting |
|
Leak at detector |
Detector-seal failure |
Replace detector seal or gaskets. |
|
Leak at injection valve |
Worn or scratched valve rotor |
Replace valve rotor |
|
Leak at pump |
Pump seal failure |
Replace pump seal; check piston for scratches and, if necessary, replace |
Change in Retention time
|
Problem |
Possible cause |
Solution |
|
Changing Retention Times |
Buffer retention times |
Use buffer with concentration greater than 20 mM. |
|
Contamination buildup |
Flush column occasionally with strong solvent |
|
|
Equilibration time insufficient for gradient run or changes in isocratic mobile phase |
Pass at least 10 column volumes through the column for gradient regeneration or after solvent changes |
|
|
First few injections - active sites |
Condition column by injecting concentrated sample |
|
|
Inconsistent on-line mobile-phase mixing |
Ensure gradient system is delivering a constant composition; compare with manually prepared mobile phase; partially premix mobile phase |
|
|
Selective evaporation of mobile-phase component |
Cover solvent reservoirs; use less-vigorous helium purging; prepare fresh mobile phase |
|
|
Varying column temperature |
Thermostat or insulate column; ensure laboratory temperature is constant. |
|
|
Decreasing Retention Times |
Active sites on column packing |
Use mobil-phase modifier, competing base (basic compounds), or increase buffer strength; use higher coverage column packing. |
|
Column overloaded with sample |
Decrease sample amount or use larger-diameter column. |
|
|
Increasing flow rate |
Check and reset pump flow rate. |
|
|
Loss of bonded stationary phase or base silica |
Use mobile-phase pH between pH 2 and pH 8 |
|
|
Varying column temperature |
Thermostat or insulate column; ensure laboratory temperature is constant |
|
|
Increasing Retention Times |
Decreasing flow rate |
Check and reset pump flow rate; check for pump cavitation; check for leaking pump seals and other leaks in system |
|
Changing mobile-phase composition |
Cover solvent reservoirs; ensure that gradient system is delivering correct composition. |
|
|
Loss of bonded stationary phase |
Use mobile-phase pH between pH 2 and pH 8 |
|
|
Slow column equilibration time |
Reversed phase ion pairing - long chain ion pairing reagents require longer equilibration time |
Use ion-pairing reagent with shorter alkyl chain length |
Baseline
|
Problem |
Possible cause |
Solution |
|
Void Time noise |
Air bubbles in mobile phase |
Degas or use back pressure restricor on detector |
|
Positive-negative - difference in refractive index of injection solvent and mobile phase |
Normal with many samples; use mobile phase as sample solvent |
|
|
Drifting baseline |
Negative direction (gradient elution) - absorbance of mobile-phase A |
Use non-UV absorbing mobile phase solvents; use HPLC grade mobile phase solvents; add UV absorbing compound to mobile phase B. |
|
Positive direction (gradient elution) - absorbance of mobile phase B |
Use higher UV absorbance detector wavelength; use non-UV absorbing mobile phase solvents; use HPLC grade mobile phase solvents; add UV absorbing compound to modile phase A. |
|
|
Positive direction - contamination buildup and elution |
Flush column with strong solvent; clean up sample; use HPLC grade solvents |
|
|
Wavy or undulating - temperature changes in room |
Monitor and control changes in room temperature; insulate column or use column oven; cover refractive index detector and keep it out of air currents. |
|
|
(11)Baseline noise |
Continous - detector lamp problem or dirty cell |
Replace UV lamp( each should last 2000 h; clean and flush flow cell. |
|
Gradient or isocratic proportioning - lack of solvent mixing |
Use proper mixing device; check proportioning precision by spiking one solvent with UV absorbing compound and mointor UV absorbance detector outputl. |
|
|
Gradient or isocratic proportioning - malfunctioning proportioning valvesl |
Clean or replace proportioning precision valves; partially remix solventsl. |
|
|
Occasional sharp spikes - external electrical interference |
Use voltage stabilizer for LC system; use independent electrical circuit. |
|
|
Periodic - pump pulses |
Service or replace pulse damper; purge air from pump; clean or replace check valves. |
|
|
Random - contamination buildup |
Flush column with strong solvent; clean up sample; use HPLC grade solvent |
|
|
Spikes - bubble in detector |
Degas mobile phase; use back pressure restrictor at detector outlet. |
|
|
Spikes - column temperature higher than boiling point of solvent |
Use lower column temperature. |
Pressure
|
Problem |
Possible cause |
Solution |
|
Decreasing Pressure |
Insufficient flow from pump |
Loosen cap on mobile phase reservior |
|
Leak in hydralic lines from pump to column |
Tighten or replace fittings; tighten rotor in injection valve |
|
|
Leaking pump check valve or seals |
Replace or clean check valves; replace pump seals. |
|
|
Pump cavitation |
Degas solvent; check for obstruction in line from solvent reservoir to pump; replace inlet-line frit |
|
|
Fluctuating pressurre |
Bubble in pump |
Degas solvent; purge solvent with helium |
|
Leaking pump check valve or seals |
Replace or clean check valves; replace pump seals |
|
|
High Back Pressure |
Column blocked wth irreversibly adorbed sample |
Improve sample cleanup; use guard column; reverse-flush column with strong solvent to dissolve blockage |
|
Column particle size too small (for example 3 micrometers) |
Use larger particle size (for example 5 micrometer) |
|
|
Microbial growth on column |
Use at least 10% organic modifier in mobile phase; use fresh buffer daily; add 0.02% sodium azide to aqueous mobile phase; store column in at least 25% organic solvent without buffer |
|
|
Mobile phase viscosity too high |
Use lower viscosity solvents or higher temperature |
|
|
Plugged frit in in-line filter or guard column |
Replace frit or guard column |
|
|
Plugged inlet frit |
Replace endfitting or frit assembly |
|
|
Polymetric columns - solvent change causes swelling of packing |
Use correct solvent with column; change to proper solvent compositionl consult manufacturer's solvent-compatibility chartl use a column with a higher percentage of cross-linking |
|
|
Salt precipitation (especially in reversed-phase chromatography with high concentration of organic solvent in mobile phase) concentration of organic solvent in mobile phase) |
Ensure mobile phase compatibility with buffer concentration; decrease ionic strength and water-organic solvent ratio; premix mobile phase |
|
|
When injector disconnected from column - blockage in injector |
Clean injector or replace rotor |
|
|
Increasing Pressure |
Blocked flow lines |
Systematically disconnect components from detector end to column end to find blockage; replace or clean blocked component |
|
Particulate buildup at head of column |
Filter sample; use .5 micrometer in-line filter; disconnect and backflush column; replace inlet frit |
|
|
Water-organic solvent systems - buffer precipitation |
Ensure mobile phase compatibility with buffer concentration; decrease ionic strength or water organic solvent ratio |
Peaks
|
Problem |
Possible cause |
Solution |
|
|
Broad peaks |
Analytes eluted early due to sample overload |
Dilute sample 1:10 and reinject |
|
|
Detector-cell volume too large |
Use smallest possible cell volume consistent with sensitivity needs; use detector with no heat exchanger in system |
||
|
Injection volume too large |
Decrease solvent strength of injection solvent to focus solute; inject smaller volume |
||
|
Large extra column volume |
Use low- or zero-dead-volume endfittings and connectors; use smallest possible diameter of connecting tubing (<0.10 in. i.d.); connect tubing with matched fittings |
||
|
Mobile-phase solvent viscosity too high |
Increase column temperature; change to lower viscosity solvent |
||
|
Peak dispersion in injector valve |
Decrease injector sample loop size; introduce air bubble in front and back of sample in loop |
||
|
Poor column efficiency |
Use smaller-particle-diameter packing, lower-viscosity mobile phase, higher column temperature, or lower flow rate |
||
|
Retention time too long |
Use gradient elution or stronger isocratic mobile phase |
||
|
Sampling rate of data system too low |
Increase sampling frequency. |
||
|
Slow detector time constant |
Adjust time constant to match peak width |
||
|
Some peaks broad - late elution of analytes retained from previous injection |
Flush column with strong solvent at end of run; end gradient at higher solvent concentration |
||
|
Ghost peaks |
Contamination |
Flush column to remove contaminatint; use HPLC-grade solven |
|
|
Elution of analytes retained from previous injection |
Flush column with strong solvent at end of run; end gradient at higher solvent concentration |
||
|
Ion-pair chromatography - upset equilibrium |
Prepare sample in mobile phase; reduce injection volume |
||
|
Oxidation of trifluoroacetic acid in peptide mapping |
Prepare trifluoroacetic acid solutions fresh daily; use antioxidant |
||
|
Reversed-phase chromatography - contaminated water |
Check suitability of water by running different amounts through column and measure peak height of interferences as function of enrichment time; clean water by running it through old reversed-phase column; use HPLC-grade water. |
||
|
Unknown interferences in sample |
Use sample cleanup or prefractionation before injection. |
||
|
Negative peaks |
Refractive index detection - refractive index of solute less than that of mobile phase |
Reverse polarity to make peak positive |
|
|
UV-absorbance detection - absorbance of solute less than that of mobile phase |
Use mobile phase with lower UV absorbance; if recycling solvent, stop recycling when recycled solvent affects detection |
||
|
Peak Doubling |
Blocked Frit |
Replace or clean frit; install 0.5-um porosity in-line filter between pump and injector to eliminate mobile-phase contaminants or between injector and column to eliminate sample contaminants |
|
|
Coelution of interfering compound |
Use sample cleanup or prefractionation; adjust selectivity by changing mobile or stationary phase |
||
|
Coelution of interfering compound from previous injection |
Flush column with strong solvent at end of ran; end gradient at higher solvent concentration |
||
|
Column overloaded |
Use higher-capacity stationary phase; increase column diameter; decrease sample amount |
||
|
Column void or channeling |
Replace column, or, if possible, open top endfitting and clean and fill void with glass beads or same column packing; repack column |
||
|
Injection solvent too strong |
Use weaker injection solvent or stronger mobile phase |
||
|
Sample volume too large |
Use injection volume equal to one-sixth of column volume when sample prepared in mobile phase for injection |
||
|
Unswept injector flow path |
Replace injector rotor |
||
|
Peak Fronting |
Channeling in column |
Replace or repack column |
|
|
Column overloaded |
Use higher-capacity stationary phase; increase column diameter; decrease sample amount |
||
|
Tailing Peaks |
Basic solutes - silanol interactions |
Use competing base such as triethylamine; use a stronger mobile phase; use base-deactivated silica-based reversed-phase column; use polymeric column |
|
|
Beginning of peak doubling |
See peak doubling |
||
|
Chelating solutes - trace metals in base silica |
Use high purity silica-based column with low trace-metal content; add EDTA or chelating compound to mobile phase; use polymeric column |
||
|
Silica-based column - degradation at high pH |
Use polymeric, sterically protected, or high-coverage reversed-phase column; install silica gel saturatorcolumn between pump and injector |
||
|
Silica-based column - degradation at high temperature |
Reduce temperature to less than 50 C |
||
|
Silica-based column - silanol interactions |
Decrease mobile-phase pH to suppress silanol ionization; increase buffer concentration; derivatize solute to change polar interactions |
||
|
Unswept dead volume |
Minimize number of connections; ensure injector rotor seal is tight; ensure all compression fittings arecorrectly seated |
||
|
Void formation at head of column |
Replace column, or, if possible, open top endfitting and clean and fill in void with glass beads or samecolumn packing; rotate injection valve quickly; use injection valve with pressure bypass; avoid pressure shock |
||
|
Spikes |
Bubbles in mobile phase |
Degas mobile phase; use back-pressure restrictor at detector outlet; ensure that all fittings are tight |
|
|
Column stored without caps |
Store column tightly capped; flush reversed-phase columns with degassed methanol |
CONCLUSION
GC and HPLC both have a place in research, product assessment, and environmental monitoring. While GC works best with analytes below 1000 daltons, HPLC is suited to separating higher molecular weight compounds in order to provide qualitative and quantitative information. HPLC chromatographic separations and separatory systems are characterized by the retention and resolution of analyte peaks, as well as their selectivity and plate number (efficiency). Hence the precision and accuracy needed for the assessment of the substances throh HPLC solely depends on the instrument and the ability to rectify the instrumentalproblems.
REFERENCE
INTERNET
1.waters.com visited on 11 november 2011 at 3:00 pm Kolkata.
2. sigmaldrich.com visited on 11 november 2011 at 3:35pm Kolkata
3. google.books.co.in visited on 12 november 2011 at 11:00 am at Kolkata.
4. forumsci.com visited on 12 november 2011 at 11: am at Kolkata.
BOOKS
5. Miachel w.Dong. Modern HPLC for practicing Scientist. Page no.275.10 second edition.
6. Stravos Kromidas ,HPLC Made to Measure ,page no.753, first edition.
7. Satinder Ahuja ,A Handbook of Pharmaceutical Analysis, page no.255, third edition.
8. Gunter J.Eppert,HPLC Trouble shooting,pageno.98 third edition.
9. Lylod .R.Sinder, John W Dolan, High Performance Gradient Elution Practical Approach to HPLC page no.153 third edition.
10. Dean Rood ,A Practical Guide to the Care and Maintenance of HPLC.first edition.
11. Journal of High Resolution Chromatography VOL 20.Page 120.second edition.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









