About Authors:
Chauhan M.K., Kawadkar J., Kishore R.*, Pathak A.M.
Department of Pharmaceutics
DIPSAR, New Delhi
*rajkishor.aryan@gmail.com
ABSTRACT
This paper discussed the problems associated with nasal drug delivery and how it is possible, sometimes by means of quite simple concepts, to improve transport across the nasal membrane. It also described the advantages, barriers, physicochemical factors, and formulation related parameters that affecting the nasal drug delivery and the applications of nasal route for delivery of peptides, proteins, non-peptide drugs, and vaccines. In this way it is feasible to deliver efficiently challenging drugs such as small polar molecules, peptides and proteins and even the large proteins and polysaccharides used in vaccines or DNA plasmids exploited for DNA vaccines. The transport of drugs from the nasal cavity directly to the brain is also described. Nasal vaccines offer several benefits, such as low enzymatic degradation compared to oral vaccines, and greater acceptability to patients. Nasal vaccines, however, have to overcome several limitations, including mucociliary clearance. Therefore, nasal vaccines require potent adjuvants and delivery systems to enhance their immunogenicity and to protect their antigens.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1323
INTRODUCTION
The last 2 decades heralded a number of advances in pharmaceutical biotechnology resulting in possibilities for large scale productions of biopharmaceuticals especially proteins and peptides. To administer these drugs scientists are moved towards drug delivery through nose. A hurdle was faced in large molecules due to short residence time of the formulation but fewer amounts in case of small organic molecules, hastens the development in number of products in market(Behl et al., 1998).
In addition, many of the advanced molecules being developed by biotechnology companies require more efficient delivery than that offered by customary routes and systems. The challenge of optimising the bio-availability and patient compliance of these expensive molecules are other significant drivers for the development of novel drug delivery systems.
The use of the nasal route for the delivery of challenging drugs has created much interest in recent years in the pharmaceutical industry. Consequently, drug delivery companies are actively pursuing the development of novel nasal drug-delivery systems and the exploitation of these for administration of conventional generic drugs and peptides, both in-house and with partners in the pharmaceutical industry. This review sets out to discuss some new developments and strategies in nasal drug delivery. An exiting discovery that drugs can be transported directly from nose to brain via the olfactory pathway is discussed and examples of proof-of-concept in man are given (Illum et al., 1998).
NASAL ANATOMY AND PHYSIOLOGY
The nasal cavity is divided into two halves by the nasal septum and extends posteriorly to the nasopharynx, while the most anterior part of the nasal cavity, the nasal vestibule, opens to the face through the nostril (Fig. 1). The atrium is an intermediate region between the vestibule and the respiratory region. The respiratory region, the nasal conchae or turbinates, which occupies the major part of the nasal cavity, possesses lateral walls dividing it into 3 sections: the superior, middle and inferior nasal turbinates. These folds provide the nasal cavity with a very high surface area compared to its small volume. The epithelial cells in the nasal vestibule are stratified, squamous and keratinized with sebaceous glands. Due to its nature, the nasal vestibule is very resistant to dehydration and can withstand noxious environmental substances and limits permeation of substances. The atrium is a transitional epithelial region with stratified, squamous cells anteriorly and pseudostratified columnar cells with microvilli posteriorly. Pseudostratified columnar epithelial cells (Fig. 2) interspersed with goblet cells, seromucus ducts, the openings of subepithelial seromucus glands cover the respiratory region (the turbinates). Furthermore, many of these cells possess actively beating cilia with microvilli. Each ciliated cell contains about 100 cilia, while both ciliated and nonciliated cells possess about 300 microvilli each. (Arora et al., 2002)
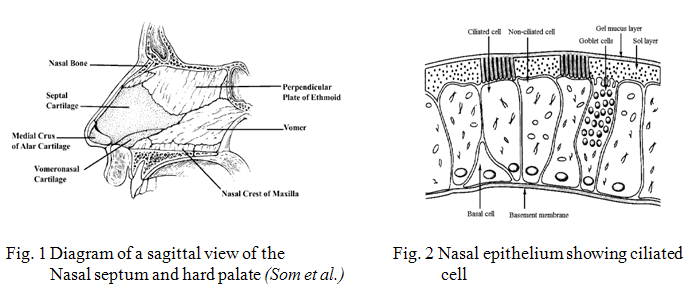
The nasal absorption of drugs is considered mainly to take place in the respiratory region comprising the turbinates and part of the nasal septum. As is the case for all biological membranes, drugs can cross the nasal mucosal membrane using two different pathways; transcellularly – across the cell – and paracellularly – between the cells. Lipophilic drugs are transported transcellularly by an efficient concentration-dependent passive diffusion process, by receptor or carrier mediation and by vesicular transport mechanisms. Polar drugs are believed to pass through the epithelium via the gaps or pores between the cells (the tight junctions). Although, the tight junctions are dynamic structures that can open and close to a certain extent, the size of these channels is less than 10 Å (McMartin et al., 1987). Hence, the paracellular route will be less efficient for large molecules and is dependent upon the molecular weight of the drug with a general molecular size cut-off of 1000 Daltons (McMartin et al., 1987).
[adsense:468x15:2204050025]
The mucociliary clearance system provides the human organism with an efficient defence system, which protects the respiratory system against inhaled bacteria, irritants and particles. It transports such agents (sticking to the viscous mucus) backwards in the nose and down into the throat. The transport of mucus is closely correlated to the beat of the cilia present on the respiratory epithelial cells. The tips of the outstretched cilia carry the viscous mucus forward with the forward stroke while on the backward stroke the cilia are bent and the movement is solely in the pericellular fluid underneath the viscous mucus. With a beat of ~1000 strokes per min, the cilia transport the mucus with a speed of 5 mm per min and formulations administered on the human respiratory epithelium has been found to be cleared from the nasal cavity with a half-life of clearance of about 15 min (Soane et al., 1999).
It is evident that for polar drugs, which are not easily transported across the nasal membrane, the mucociliary clearance mechanism can quickly move the drug away from the absorption site in the nasal cavity into the oesophagus, whereby the drug is swallowed and the absorption minimized (Illum et al., 1998).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MERITS OF NASAL DRUG DELIVERY SYSTEMS
1) Easy accessibility and needle free drug application without the necessity of trained personnel facilitates self medication, thus improving patient compliances compared to parenteral routes (Pontiroli et al., 1991).
2) Good penetration of, especially lipophilic, low molecular weight drugs through the nasal mucosa. For instance the absolute nasal bioavailability of fentanyl is about 80% (Striebel et al., 1993).
3) Rapid absorption and fast onset of action due to relatively large absorption surface and high vascularization. Thus the Tmax of fentanyl after nasal administration was less than or equal to 7 minute comparable to intravenous (Striebel et al., 1993). Nasal administration of suitable drug would therefore be effective in emergency therapy as an alternative to parenteral administration routes.
4) Avoidance of the harsh environmental conditions in the gastrointestinal tract (chemical and enzymatic degradation of drugs).
5) Avoidance of hepatic first pass metabolism and thus potential for dose reduction compared to oral delivery.
6) Potential for direct delivery of drug to the central nervous system via the olfactory region, thus by-passing the blood brain barrier (Talegaonkar et al., 2004).
7) Direct delivery of vaccine to lymphatic tissue and induction of a secretary immune response at distant mucosal site (Davis et al., 2001).
IMPEDIMENT IN NASAL DRUG DELIVERY
1)Low bioavailability
Bioavailability of polar drugs is generally low, about 10% for low molecular weight drugs and not above 1% for peptides such as calcitonin and insulin (Illum et al., 2002). The most important factor limiting the nasal absorption of polar drugs and especially large molecular weight polar drugs such as peptides and proteins is the low membrane permeability. Drugs can cross the epithelial cell membrane either by the transcellular route exploiting simple concentration gradients, by receptor mediated or vesicular transport mechanisms, or by the paracellular route through the tight junctions between the cells. Polar drugs with molecular weights below 1000 Daltons will generally pass the membrane using the latter route (McMartin et al., 1987).
Although tight junctions are dynamic structures and can open and close to a certain degree when needed, the mean size of these channels is of the order of less than 10 Å and the transport of larger molecules is considerably more limited (McMartin et al., 1987).Larger peptides and proteins are able to pass the nasal membrane using an endocytotic transport process but only in low amounts (Inagaki et al., 1985).
Nasal absorption of such polar drugs can be greatly improved by co administration of absorption enhancing agents (Illum., 2000).
2)Mucociliary clearance
The general fast clearance of the administered formulation from the nasal cavity due to the mucociliary clearance mechanism is another factor of importance for low membrane transport. This is especially the case when the drug is not absorbed rapidly enough across the nasal mucosa. It has been shown that for both liquid and powder formulations, which are not bioadhesive, the half life for clearance is of the order of 15 - 30 min (Soane et al., 1999) and (Illum et al., 1999).
The use of bioadhesive excipient in the formulations is an approach to overcome the rapid mucociliary clearance. The clearance may also be reduced by depositing the formulation in the anterior, less ciliated part of the nasal cavity thus leading to improved absorption (Kublik et al., 1998) and (Harris et al., 1986).
3)Enzymatic Degradation
Another contributing, but often less considered factor to the low bioavailability of peptides and proteins across the nasal mucosa is the possibility of an enzymatic degradation of the molecule in the lumen of the nasal cavity or during passage through the epithelial barrier. These sites both contain exopeptidases such as monoand diaminopeptidases that can cleave peptides at their N and C termini and endopeptidases such as serine and cysteine, which can attack internal peptide bonds (Lee., 1988).
LIMITATION NASAL DRUG DELIVERY SYSTEMS
(Kadam et al., 1993 and Hirai et al., 1981)
1) The absorption enhancers used to improve nasal drug delivery system may have histological toxicity which is not yet clearly established.
2) Absorption surface area is less when compared to GIT.
3) Once the drug administered can not be removed.
4) Nasal irritation
MECHANISM OF DRUG ABSORPTION
The first step in the absorption of drug from the nasal cavity is passage through the mucus (Illum et al., 1999). Small, unchanged particles easily pass through this layer. However, large or charged particles may find it more difficult to cross. Mucin, the principle protein in the mucus, has the potential to bind to solutes, hindering diffusion. Additionally, structural changes in the mucus layer are possible as a result of environmental changes (i.e. pH, temperature, etc. (Illum et al., 1999). Subsequent to a drug’s passage through the mucus, there are several mechanisms for absorption through the mucosa (Illum et al., 1999). These include transcellular or simple diffusion across the membrane, paracellular transport via movement between cell and transcytosis by vesicle carriers (Illum et al., 1999). Obstacles to drug absorption are potential metabolism before reaching the systemic circulation and limited residence time in the cavity. Several mechanisms have been proposed but the following two mechanisms have been considered predominantly.
1) The first mechanism involves an aqueous route of transport, which is also known as the paracellular route. This route is slow and passive. There is an inverse log-log correlation between intranasal absorption and the molecular weight of water-soluble compounds. Poor bioavailability was observed for drugs with a molecular weight greater than 1000 Daltons (Aurora, 2002).
2) The second mechanism involves transport through a lipoidal route that is also known as the transcellular process and is responsible for the transport of lipophilic drugs that show a rate dependency on their lipophilicity. Drugs also cross cell membranes by an active transport route via carrier-mediated means or transport through the opening of tight junctions. For example, Chitosan, a natural biopolymer from shellfish, opens tight junctions between epithelial cells to facilitate drug transport (Dodane et al., 1999).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
INFLUENCING FACTORS
1) On Mucoadhesion of Polymer
The factors that influence mucoadhesiveness of a polymer include type of functional groups present, polymer molecular mass, molecular mass between cross-links (cross- linking density), spatial orientation, contact time with mucus, polymer concentration, environmental pH and physiological variables like mucin turnover and disease conditions. The polymer molecular mass will influence its bioadhesion characteristics. There is a critical polymer molecular mass and cross-linking density below or above which there is reduced adhesive power, and this varies with the type of polymer (Tobyn et al., 1996), (Gurny et al., 1984) and (Blanco-Fuente et al., 1996).
Mucoadhesion requires an adequate free chain length for interpenetration to occur. Reducing the free chain length by extensive cross-linking therefore reduces mucoadhesion (Park et al., 1987).
An optimum polymer concentration is required at the polymer mucus interface for bioadhesion, beyond which few polymer chains will be available for polymer mucus interpenetration. The polymer concentration that is required for optimum bioadhesion is different between gels and solid bioadhesives. In the liquid state, an optimum concentration exists for each polymer beyond that reduced adhesion results because fewer polymer chains will be available for interpenetration with the mucus. On the other hand, with solid dosage forms such as buccal tablets, increased polymer concentration leads to increased mucoadhesive power (Duchene et al., 1988), (Ponchel et al., 1987) and (Ponchel et al., 1987).
1.1Hydration and swelling of polymer
Polymer hydration and swelling are required for initiation of mucoadhesion but excessive hydration with inordinate swelling of the polymer reduces its adhesive strength. The swelling/hydration rate should not be too rapid in order to prolong the adhesion time. On the other hand, inordinate swelling is eventually required to reduce polymer adhesiveness and to allow it to detach from the biological tissue (Park et al., 1985).
1.2 Environmental pH affects hydrogen bonding
Some polymers owe their mucoadhesiveness to such forces as hydrogen bonding, van der Waals, hydrophobic and electrostatic forces. The strength of these forces is influenced by the environmental pH. Consequently, for such polymers, environmental pH is a very important determinant of mucoadhesive strength. This has been clearly demonstrated for polycarbophil (Park et al., 1985)and more recently for chitosan (Kotze et al., 1999).
2) On Nature of Drug
2.1 Lipophilicity
On increasing lipophilicity, the permeation of the compound normally increases through nasal mucosa. Although the nasal mucosa was found to have some hydrophilic character, it appears that these mucosae are primarily lipophilic in nature and the lipid domain plays an important role in the barrier function of these membranes (Corbo et al., 1990).
In one study it was found that lipophilic compounds alprenolol and propranolol were well absorbed from the nasal mucosa, in contrast to the hydrophilic drug metoprolol. Lipophilic compounds tend to readily cross biological membranes via the transcellular route since they are able to partition into the lipid (bilayer) of the cell membrane and diffuse into and traverse the cell in the cell cytoplasm. A number of lipophilic drugs such as naloxone, buprenorphine, testosterone (Hussain et al., 1984) and 17a-ethinyloestradiol (Bawarshi et al., 1989) have been shown to be completely or almost completely absorbed nasally in animal models. A correlation between lipophilicity and nasal drug absorption has been demonstrated using several compounds (Hussain et al., 1991).
2.2 Chemical Form
The chemical form of a drug can be important in determining absorption. For example, conversion of the drug into a salt or ester form can alter its absorption (Huang et al., 1985) studied the effect of structural modification of drug on absorption. It was observed that in-situ nasal absorption of carboxylic acid esters of L-Tyrosine was significantly greater than that of L-Tyrosine.
2.3 Polymorphism
Polymorphism is known to affect the dissolution rate and solubility of drugs and thus their absorption through biological membranes. It is therefore advisable to study the polymorphic stability and purity of drugs for nasal powders and/or suspensions.
2.4 Molecular Weight
In the case of lipophilic compounds, a direct relationship exists between the MW and drug permeation whereas water soluble compounds depict an inverse relationship. Based on the reports by (Fisher et al. 1992)and (Yamamto et al.1993), it can be concluded that the permeation of drugs less than 300 Daltons is not significantly influenced by the physicochemical properties of the drug, which will mostly permeate through aqueous channels of the membrane. By contrast, the rate of permeation is highly sensitive to molecular size for compounds with MW ≥ 300 Daltons.
2.5 Partition Coefficient and pKa
As per the pH partition theory, unionized species are absorbed better compared with ionized species and the same holds true in the case of nasal absorption (Jiang et al.1997) conducted a study to determine the quantitative relationship between the physicochemical properties of drugs and their nasal absorption, using diltiazem hydrochloride and paracetamol as model drugs. The results showed that a quantitative relationship existed between the partition coefficient and the nasal absorption constant. The nasal absorption of weak electrolytes such as salicylic acid and aminopyrine was found to be highly dependent on their degree of ionization. Although for aminopyrine, the absorption rate increased with the increase in pH and was found to fit well to the theoretical profile, substantial deviations were observed with salicylic acid. The authors concluded that perhaps a different transport pathway, along with the lipoidal pathway, existed for salicylic acid (Hirai et al., 1981). Similarly, when the absorption of benzoic acid was studied at pH 7.19 (99.9% of the drug existed in ionized form) it was found that 10% of drug was absorbed indicating that the ionized species also permeates through nasal mucosa (Huang et al., 1985). Based on all of these observations, the authors accounted partition coefficients as a major factor governing nasal absorption and supported that other transport pathways for hydrophilic drugs might be of importance.
2.6 Solubility & Dissolution Rate
Drug solubility and dissolution rates are important factors in determining nasal absorption from powders and suspensions. The particles deposited in the nasal cavity need to be dissolved prior to absorption. If a drug remains as particles or is cleared, no absorption takes place.
3) On Nature of Formulation
Physicochemical Properties of the Formulation
3.1 pH and Mucosal Irritancy
The pH of the formulation, as well as that of nasal surface, can affect a drug’s permeation. To avoid nasal irritation, the pH of the nasal formulation should be adjusted to 4.5–6.5 (Arora et al., 2002). In addition to avoiding irritation, it results in obtaining efficient drug permeation and prevents the growth of bacteria. As shown in Fig 3.
3.2 Osmolarity
Ohwaki et al. studied the effect of osmolarity on the absorption of secretin in rats and found that absorption reached a maximum at a sodium chloride concentration of 0.462 M (Ohwaki et al., 1985), because shrinkage of the nasal epithelial mucosa was observed at this salt concentration (Ohwaki et al., 1987). This results in increased permeation of the compound resulting from structural changes and was further confirmed when sorbitol was used as an osmoregulatory agent. The authors found that permeation of secretin subsequently decreased (Ponchel et al., 1987)and, therefore, isotonic solutions are usually preferred for administration.
3.3 Viscosity
A higher viscosity of the formulation increases contact time between the drug and the nasal mucosa thereby increasing the time for permeation. At the same time, highly viscous formulations interfere with the normal functions like ciliary beating or mucociliary clearance and thus alter the permeability of drugs.
4) On Behaviour of Physiology of Nasal Area
4.1 Blood flow related
Nasal absorption of drugs is influenced by blood flow rate, as it increases the amount of drug that passes through the membrane and hence reaching the general circulation. Several studies were made to evaluate this influence. For example, Kao et al. stated that nasal absorption of dopamine was relatively slow and incomplete probably due to its own vasoconstrictor effect. From above observations, it was concluded that vasoconstriction decreases nasal drug absorption by diminishing the blood flow. (Kao et al., 2000).
4.2 Mucociliary clearance (MCC) related
MCC, mucus turnover and disease states are physiological factors which influence nasal mucoadhesion. Mucoadhesion can slow down MCC, but with time, mucus production reduces the mucoadhesion bond strength, allowing a recovery of MCC to normal clearance rates, thereby removing the mucoadhesive. Disease conditions mentioned earlier can affect mucoadhesion due to their influence on either mucus production or ciliary beating. Thus a good understanding of the nature of mucus in these diseases is imperative in designing a good NDD system. An abnormal mucus layer could present an unanticipated barrier to drug transport through the mucosa. Mucoadhesive capabilities of polymers should be studied during product development under such disease conditions considered relevant.
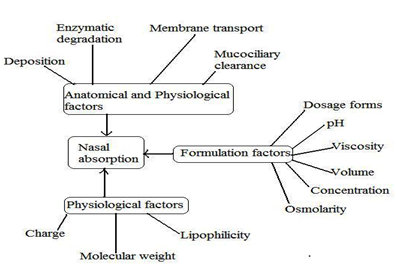
Fig 3:The physicochemical, anatomical, physiological and formulation factors affecting the nasal absorption of drugs.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
DEVELOPMENTS
Potential opportunities for introducing nasal delivery exert its prime function as a filter and air-conditioner protecting the lower airways, the nose has a complex geometry lined by highly vascularised mucosa.The easy access to this large vascularised surface makes the nose particularly attractive for absorption of drugs which are difficult to deliver with conventional methods and normally require injection. Rapid absorption and the fast onset of action are essential in treating intense, acute pain and in management of severe events such cardiovascular attacks, seizures, hypoglycemia, nausea and vomiting. Nasal administration limits the problems associated with degradation of drugs in the stomach and in the liver, which makes it particularly relevant for many of the new recombinant peptide and protein drugs. The nasal route provides an attractive needle-free alternative which may improve patient compliance and allow extended use of self-medication for many chronic diseases. As shown in Fig 4.
1) Intranasal Vaccination
Nasal mucosa is also extremely rich in specialized cells and houses organized lymphatic tissues involved in the first line defense against airborne micro-organisms. Nasal vaccination avoids the discomfort and a problem associated with injection, and stimulates local mucosal defense as well as a systemic immune response. In addition, nasal vaccination induces protection in distant mucosal organs and appears to provide broader protection than injected vaccines. Several nasal vaccines are in the pipeline and expected to enter the market in the near future (Djupesland P.G., 2003).
The nasal area with its nose associated lymphoid tissue (NALT) is also an inductive as well as an effective site of the immune system (Kuper et al., 1992). In humans the NALT is known as the Waldeyer´s Ring, Nasal secretions are known to contain immunoglobulins (IgA, IgG,IgM, IgE), protective proteins such as complement as well as neutrophils and lymphocytes in the mucosa (Durrani et al., 19980). Delivering the vaccine to the nasal cavity itself stimulates the production of local secretary IgA antibodies as well as IgG, providing an additional first line of defense, which helps to eliminate the pathogen before it becomes established.
Many diseases, such as measles, pertussis, meningitis and influenza are associated with the entry of pathogenic microorganisms across the respiratory mucosal surfaces and are hence good candidates for nasal vaccines. It is well established that nasally administered vaccines, especially if based on attenuated live cells or adjuvanted by means of an immunostimulator or a delivery system, can induce both mucosal and systemic (i.e. humoral and cell-mediated) immuneresponses. The feasibility of the nasal route for administering vaccines against plague, diphtheria tetanus (Alpar et al., 2001), influenza (Singh et al., 2001), cholera (yuki et al., 2001) and HIV (Velin et al., 2000) has already been tested for inducing both mucosal and systemic immune response against the carried antigen (Mikszta et al.)have developed a new anthrax vaccine which is based on recombinant Bacillus anthracis protective antigen (rPA). They investigated micro needle-based cutaneous and nasal mucosal delivery of rPA in mice and rabbits. In mice, intra-dermal (id) delivery achieved up to 90% seroconversion after a single dose, compared with 20% after intra-muscular (im) injection. Intranasal (inl) delivery of a liquid formulation required 3 doses to achieve responses that were comparable with those achieved via the id or intramuscular routes. In rabbits, id delivery provided complete protection against aerosol challenge with anthrax spores; in addition, novel powder formulations administered (inl) provided complete protection, whereas a liquid formulation provided only partial protection. These results demonstrate, for the first time, that cutaneous or nasal mucosal administration of rPA provides complete protection against inhalation anthrax in rabbits. The novel vaccine/device combinations described here have the potential to improve the efficacy of rPA. (Read et al., 2005) have prepared nasal influenza vaccine using chitosan. Nasal influenza vaccination may prove to be a good alternative to parenteral injection because of the enhancement of the mucosal immune response and the ease of vaccine administration. This study investigated the use of chitosan, a bioadhesive polymer, as a nasal delivery system with inactivated, subunit influenza vaccine. Subjects received nasally 15 or 7.5 g of the standard inactivated trivalent influenza vaccine with chitosan or 15 g of the same vaccine intramuscularly. Serum haemagglutination inhibition (HI) titres for all three vaccine components were measured prior to, and at time points up to 14 weeks after dosing. Serum HI titres following intranasal vaccination with the nasal chitosan influenza vaccine met the criteria set by the Committee for Proprietary Medicinal Products in terms of seroprotection rate, seroconversion rate and mean fold increase of HI titre for at least one of the three antigens in the vaccination schedules used. These data show that nasal immunizaimmunization with chitosan plus trivalent inactivated influenza is a potentially effective, easily-administered form of vaccination.
2) Nose to Brain Delivery
The nose to brain delivery would be beneficial in therapeutic situations where a rapid and/or specific targeting of drugs to the brain is required. Conditions such as Parkinson’s disease, Alzheimer’s disease or pain would be benefited from the development of nasal delivery systems, which will increase the fraction of drug that reach the CNS after nasal delivery. The olfactory region located at the upper remote parts of the nasal passages offers the potential for certain compounds to circumvent the blood-brain barrier and enter into the brain. Neurotrophic factors such as NGF (Frey et al., 1997), (Chen et al., 1998), (Frey et al., 2000) IGF-I (Kucheryanu et al., 1999) FGF (Gozes et al., 1996) and DNF (Pietrowsky et al., 1996) have been intranasally delivered to the CNS in rodents. Studies in humans, with proteins such as AVP (Pietrowsky et al., 1996), CCK analog (Smolnik et al., 1999), MSH/ACTH (Fehm et al., 2001) and (Kern et al., 1999) and insulin (Hinchcliffe et al., 1999) and (Quay et al., 2001) have revealed that they are delivered directly to the brain from the nasal cavity. (Frey et al., 2000) declared that by nasally administering insulin like growth factor (IGF-1) the drug could bypass the blood brain barrier and reach the central nervous system directly from the nasal cavity. Reports in the literature of studies in animal models and in man have shown this to be a distinct possibility with results showing the uptake of drugs into the cerebrospinal fluid and the brain tissue being dependent upon molecular weight and the lipophilicity (Illum., 2000), (Fehm et al., 2000) and (Perras et al., 1999). Various studies in animal models have confirmed that, at early time points after nasal administration, the concentration of cocaine in the brain was higher after nasal administration than after intravenous administration, thereby showing the existence of a pathway from the nose to the brain (Chow et al., 1999). (Dahlin et al. 2000) carried a study to investigate whether dopamine is transferred along the olfactory pathway to the brain following nasal administration to mice. [3H]-Dopamine was administered nasally or intravenously to female mice. Brain tissue samples were excised and the radioactive content was measured. The precise localization of dopamine radioactivity in the brain was studied using autoradiography. The presence of dopamine or its metabolites in the olfactory bulb and mucosa was ascertained using thin layer chromatography (TLC).The results indicate that unchanged dopamine is transferred into the olfactory bulb following nasal administration of [3H]-dopamine. Kumar et al. have studied the nasal uptake of tritium labeled estradiol and progesterone in rhesus monkeys. Both compounds were found to be absorbed intranasally and were able to penetrate into cerebrospinal fluid rapidly. A comparison of the plasma:CSF ratio of the two compounds indicated more steroids accumulated in the CSF after ability and minimizing variation. They demonstrated intranasal administration compared to the intravenous route. This result suggested that the two steroids can be absorbed from the nasal cavity and reach the brain directly via the respiratory and olfactory mucosa. (Sakane et al., 1991)reported that following intranasal administration of the antibiotic cephalexin to rats, higher CSF concentration was reached at 15 min but it declined to approximately half that concentration at 30 min. Because cephalexin did not cross the BBB well and CSF concentration was 166-fold higher after intranasal administration than after systemic administration in spite of similar blood levels, it was concluded that cephalexin entered the CSF directly from the nasal cavity. Using a series of fluorescein isothiocyanate labeled dextrans (FITC-dextran) with increasing molecular weights, it was found that dextrans with molecular weights of upto 20,000 daltons could be transported directly from the nasal cavity of rats into the CSF. The concentration of the FITC-dextrans in the CSF increased with decreasing molecular weight. These FITC dextrans were not found in the CSF after intravenous administration. Recently, (Shi et al., 2005) investigated the extent of systemic absorption and uptake of meptazinol (MEP) hydrochloride in cerebrospinal fluid (CSF) after intranasal administration on rats and compared with oral administration. CSF samples were collected by a serial sampling method. The concentration of MEP in the biological samples was measured by HPLC with fluorescence detector. A rapid and significant level of MEP in the plasma and CSF was achieved after nasal administration whereas the oral administration resulted in considerably lower drug concentrations. The area under curve (AUC) in plasma and CSF from the nasal route was 7.375 and 15.6 folds compared with those of the oral route, respectively. The outcome of the research indicated that intranasal MEP is able to show quick absorption and improve the bioavailability, which could be a promising alternative to oral administration.
3) Delivery of Genes
A major clinical challenge for delivery of genes to the CNS results from the limitations of the currently available vectors. Most of the viral vectors are too big, and have to be injected directly into brain tissues. Therefore, nasal administration for delivery of plasmid DNA encoding therapeutic or antigenic genes is gaining attention in recent years as an alternative method due to its non-invasive administration.
The beta-galactosidase protein encoded by the recombinant plasmids was significantly expressed in brain tissues following intranasal administration. Over 1 hour after dosing, the brain targeting efficiencies were shown consistently higher for plasmid DNA administered intranasally than that administered intravenously. The authors concluded that intranasally applied plasmid DNA may reach the brain through a direct route, possibly via the olfactory bulb, and that the nasal route might be an alternative method to deliver plasmid DNA to the brain (Han et al., 2007).
The advantage of the vectors based on herpes simplex virus is that they are neurotropic. However, previous study has reported that vectors based on the type 1 herpes simplex virus (HSV-1) induced apoptosis in CNS neurons, causing severe and often fatal encephalitis in immunocompetent humans (Perkins et al., 2003). However, vectors based on herpes simplex type 2 virus, RR, is less virulent in the CNS than HSV-1, and it does not trigger apoptosis in CNS neurons (Perkins et al., 2003). A study showed that RR delivered by intranasal route protected rats and mice from seizures and neuronal loss, granting it as a promising therapeutic platform for the treatment of chronic neurodegenerative diseases (Laing et al., 2006).
One study investigated the intranasal delivery of calcitonin gene-related peptide (CGRP), a potent vasodilator, to the brain. The data suggested that intranasal CGRP significantly relieved vasospasm, improved cerebral blood flow, and reduced cortical and endothelial cell death. Intranasal route was shown to be an effective way to deliver CGRP for brain targeting (Sun et al., 2010).
Delivery of polynucleotide agents (e.g., naked DNA, RNA, and antisense) has already been granted patent for directly transporting along the olfactory or trigeminal neural pathways to the CNS to achieve either the controlled expression of a polypeptide or the in vivo production of an antisense polynucleotide sequence (Reinhard and Frey,2009, US Patent 12419999).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4) Nasal Mucoadhesive Delivery of Pharmaceutical Compounds
4.1 Liposomes
Liposomes are soft vesicular structures formed by self-assembly of phospholipids which are the same materials as cell membranes. They can be formed in many shapes and sizes depending on lipid composition. Liposomes are often used as non-viral carriers for DNA delivery because of their dynamic properties of cellular membranes that interact with the biological environment (Balazs et al., 2011).
Cationic liposomes were able to enhance the interferon-inducing and antiviral activity of ridostin (an interferon inducer) in experiments with cell cultures of L-929 (Bulychev et al., 2003). Liposomes can also be coated with several thousand strands of polyethylene glycol (PEG) to extend the circulation time in the blood. About 1-2% of the PEG polymer tips are conjugated with a targeting monoclonal antibody which acts as a molecular Trojan horse, specific to brain receptor. This type of Trojan horse liposome is also called PEGylated immunoliposomes. The molecular Trojan horse then binds to a receptor on the BBB and brain cell membrane, triggering receptor-mediated transcytosis of the liposome across the BBB, and endocytosis into brain cells (Pardridge, 2010).
4.2 Nanoparticles
Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting has shown increased drug delivery to the brain (Al-Ghananeem et al., 2010). Despite the positive experimental results in improving nose to brain delivery of nano sized drugs in animal studies, it is still uncertain at this stage whether drug carried by the nanoparticles is being released in the nasal cavity or the nanoparticles carrying the drug are transported via the olfactory or the trigeminal nerves into the CNS where the drug is then released (Mistry et al., 2009).
4.3 Topical Nasal Drugs
The current value of the nasal drug market is approximately US$10 billion, with an expected growth of 10-15 per cent annually. Topical decongestants and topical steroids currently account for more than two thirds of the market value. Allergies are also on the rise worldwide and affect 5-10 per cent of the population. Topical steroids represent the drugs of choice for patients with chronic allergic and non-allergic mucosal inflammation. Furthermore, the topical steroids used to treat rhinitis are also used in patients with sinusitis. Chronic rhinosinusitis and nasal polyposis are often associated with asthma and require life-long treatment. However the clinical effect of topical steroids is often disappointing, largely due to the inadequate distribution to the nose and sinuses. Thus, improved treatment modalities for chronic rhinitis and chronic sinusitis have the potential for considerable market growth in existing and new topical drugs, and open new avenues for novel delivery nasal systems able to improve patent compliance and the clinical effects (Djupesland P.G., 2003).
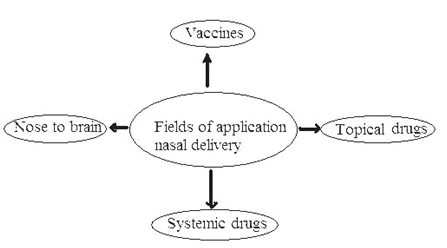
Fig 4: Fields for Application of nasal delivery
MARKETED PREPARATIONS FOR NASAL DRUG DELIVERY
Table 1: Nasal Drug Products Available in the Market
|
For Vaccination |
|||
|
S.No. |
Product name |
Dosage form |
Manufacturer
|
|
1 |
Human influenza vaccine (Nasalflu Berna) |
Virosomes (Spray) |
Berna Biotech
|
|
2 |
Human influenza vaccine (FluMist) |
Spray |
Medlmmune Inc.
|
|
3 |
Feline trivalent vaccine against calici herpes-1and parvovirus |
Drops |
Heska |
|
4 |
Equine influenza vaccine (Flu Avert) |
Drops |
Heska
|
|
5 |
Porcine Bordetella bronchiseptica vaccine (Maxi/ Guard Nasal Vac) |
Drops |
Addison Biological Laboratory |
|
6 |
Feline Bordetella bronchiseptica vaccine (Nobivac Bp) |
Suspension drops |
Intervet |
|
7 |
Human Streptococcus A vaccine (StrepAvax) |
Proteosomes (nanoparticulate) |
ID Biomedical |
|
8 |
Human influenza vaccine (FluINsuru) |
Proteosomes (nanoparticulate) |
ID Biomedical |
|
9 |
Human influenza vaccine |
Spray |
West PS |
|
10 |
Human Influenza vaccine Not indicated. Preclinical Chiron |
Spray |
Chiron |
|
Proteins and Peptides for Systemic Drug Delivery |
|||
|
1 |
Salmon calcitonin (Karil 200 I.E.) |
Solution (spray) |
Novartis Pharma
|
|
2 |
Desmopressin (Minirin asenspray) |
Solution (spray) |
Ferring Arzneimitted |
|
3 |
Buserelin (Profact nasal) |
Solution (spray) |
Aventis Pharma
|
|
4 |
Nafarelin (Synarela) |
Solution (spray) |
Pharmacia
|
|
5 |
Oxytocin (Syntocinon) |
Solution (spray) |
Novartis Pharma
|
|
6 |
Protirelin (antepan nasal) (Relefact TRH nasal) |
Solution (spray) |
Sanofi-synthelabo Aventis Pharma |
|
Non-Peptide for Systemic Drug Delivery |
|||
|
1 |
Zolmitriptan (AscoTop Nasal) |
Solution (spray) |
Astra Zeneca |
|
2 |
Sumatriptan (Imigran Nasal) |
Solution (spray) |
Glaxo SmithKline |
|
3 |
Dihyfroergotamin (Migranal NasalSpray) |
Solution (spray) |
Novartis Pharma |
|
4 |
Estradiol (Aerodiol) |
Solution (spray) |
Servier |
ADVANCES IN DELIVERY DEVICES
The increased interest in nasally delivered drugs and vaccines has, however, spurred the demand for improved nasal delivery technologies. Disposable unitdose devices reduce the problems associated with spray pump priming and hygiene but, to date, efficient and consistent distribution to the nasal mucosa has proven difficult to achieve in practice (Djupesland P.G., 2003).
Monodose / single-use devices
The single-use device concept will from a cost perspective be ideal in applications where low numbers of doses are to be delivered. These applications include for instance nasal vaccines. Also from the concern for safety and abuse it would be preferable only to apply unit dose devices for administration of for instance anti-migraine doses.
The issue of hygiene is important in nasal delivery
The wide use of nasal multi-dose delivery devices, has given rise to the need for preservatives to be added to the formulations, in order to prevent microbiological contamination of the bulk reservoir of the drug in the devices. This concerns both multidose liquid and dry powder devices. Those preservatives are incompatible with some active ingredients, and may be irritant to the nasal epithelium. Therefore, preservative-free systems are requested.
It has been argued that an individually sealed dry powder dose in multidose devices is an option for avoiding this problem. However, the only effective way to achieve completely hygienic nasal delivery is by use of single-use disposable devices. Direct Haler Nasal belongs to this new category of devices.
A single-use disposable product is the general reference in hygienic performance. Therefore, such single-use products have taken market from other products, whenever the disposables meet general requirements on performance and cost.
CONCLUSION
Nasal delivery provides a cost-effective and user-friendly alternative to injection. A drug delivered nasally act faster than tablets and mixtures, and the onset of action is comparable to intravenous injection. Intranasal vaccination offers additional local immune protection for many vaccines. The nose is an attractive delivery route worth considering for many existing substances, as well as the complex protein drugs being developed by biotechnology companies. Progress in nasal formulation technologies and new delivery technologies such as bi-directional delivery may offer essential advantages and expand the market for nasal delivery of drugs and vaccines.
REFERENCES
1. Al-Ghananeem AM, Saeed H, Florence R, Yokel RA, Malkawi AH., 2010, Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by AIDS viruses. J Drug Target, 381-388.
2. Alpar HO, Eyles JE, Williamson ED, Somavarapu S., 2001, Intranasal vaccination against plague, tetanus and diphtheria. Adv Drug Deliv Rev, 173-201.
3. Anand KTC, David GFX, Umberkoman B, Saini KD., 1974, Uptake of radioradioactivity by body fluids and tissues in rhesus monkeys after intravenous injection or intranasal spray of tritium-labelled estradiol and progesterone. Curr Sci, 435-439.
4. Arora P, Sharma S, Garg S., 2002, Permeability issues in nasal drug delivery.Drug Discov Today, 967-975.
5. Aurora J. 2002, Development of Nasal Delivery Systems: A Review.Drug Deliv Technol ,1-8.
6. Balazs DA, Godbey W. Liposomes for use in gene delivery. J Drug Deliv , 326497, 2011.
7. Bawarshi RN, Hussain A, Crooks PA., 1989, Nasal absorption of 17aethinyloestradiol in the rat. J Pharm Pharmacol, 214-215.
8. Behl C.R., Pimplaskar H.K., Sileno A.P., de Meireles J., Romeo V.D., 1998, Effects of physicochemical properties and other factors on systemic nasal drug delivery, Adv. Drug Deliv. Rev. 89– 116.
9. Blanco-Fuente H., Anguiano S., Otero-Espinar F.J., Balnco-Mendez J., 1996, In vitro bioadhesion of Carbopol hydrogels, Int. J. Pharm. 169– 174.
10. Bulychev LE, Poryvaev VD, Ryzhikov AB, Karpyshev NN, Alekseeva AG, Goncharova EP, Pliasunov IV., 2003, An intensification of antiviral and interferon effects of ridostin (an interferon inducer) by cationic liposomes in vitro and in intranasal administration. Vopr Virusol, 45-47.
11. Chen XQ, Fawcett JR, Rahman YE, Ala TA, Frey WH. 1998, Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis, 35-44.
12. Chow HS, Chen Z, Matsuura GT., 1999, Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci, 754-758.
13. Corbo DC, Liu JC, Chien YW., 1990, Characterization of the barrier properties of mucosal membranes. J Pharm Sci , 202-206.
14. D. Duchene, F. Touchard, N.A. Peppas, 1988, Pharmaceutical and medical aspects of bioadhesive systems for drug administration, Drug Dev. Ind. Pharm. 283– 318.
15. Dahlin M, Bergman U, Jansson B, Bjork E, Brittebo E., 2000, Transfer of dopamine in the olfactory pathway following nasal administration in mice. Pharm Res, 737-742.
16. Davis SS., 2001 Nasal vaccines. Adv Drug Deliv Rev , 21-42.
17. Djupesland P.G., 2003,Who Nose How Far Nasal Delivery Can Go? European Pharmaceutical Contractor Autumn 03 issue.
18. Dodane V, Khan MA, Merwin JR., 1999, Effect of chitosan on epithelial permeability and structure. Int J Pharm, 21-32.
19. Durrani Z, McInterney TL, McLain L, et al., 1998, Intranasal immunisation with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization. J Immunol Methods , 93-103.
20. Fehm HL, Perras B, Smolnik R, Kern W, Born J., 2000, manipulating neuropeptidergic pathways in humans: A novel approach to neuropharmacology. Eur J Pharmacol, 43-54.
21. Fehm HL, Smolnik R, Kern W, McGregor GP, Bickel U, Born J., 2001,The melanocortin melanocyte-stimulating hormone/adrenocotropin (4-10) decreases body fat in humans. J Clin Endocrinol Metab, 1144-1148.
22. Fisher A, Illum L, Davis S, Schacht E. 1992,Di-iodo-L-tyrosine labelled dextrans as molecular size markers of nasal absorption in the rat. J Pharm Pharmacol ,44: 550-554.
23. Frey WH, Liu J, Chen X, Thorne RG, Fawcett JR, Ala TA, 1997, Delivery of 125I-NGF to the brain via the olfactory route, Drug Delivery, 87-92.
24. Frey WH, Thorne RG, Pronk G. 2000, Delivery of Insulin like growth factor-1 to the brain and spinal cord along olfactory and trigeminal pathways following intranasal administration: a noninvasive method for bypassing the blood brain barrier. Soc Neurosci Abstract, 1365-1370.
25. Gozes I, Bardea A, Reshef A, et al. 1996, Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proc Natl Acad Sci USA , 427-432.
26. Gurny R., Meyer J.M., Peppas N.A., 1984, Bioadhesive intraoral release systems: design, testing and analysis, Biomaterials , 336–340.
27. Han IK, Kim MY, Byun HM, Hwang TS, Kim JM, Hwang KW, Park TG, Jung WW, Chun T, Jeong GJ, Oh YK. Enhanced brain targeting efficiency of intranasally administered plasmid DNA: an alternative route for brain gene therapy. J Mol Med, 75-83, 2007.
28. Harris AS, Nilsson IM, Wagner ZG, Alkner U., 1986, Intranasal administration of peptides: nasal deposition, biological response, and absorption of desmopressin. J Pharm Sci, 1085-1088.
29. Hinchcliffe M, Illum L., 1999, Intranasal insulin delivery and therapy.Adv Drug Deliv Rev, 199-234.
30. Hirai S, Yashika T, Matsuzawa T, Mima H., 1981,Absorption of drugs from the nasal mucosa of rat. Int J Pharm, 317-325.
31. Hirai S, Yashiki T, Mima H., 1981, Effect of surfactants on nasal absorption of insulin in rats, Int. J. Pharm, 165?171.
32. Huang C, Kimura R, Nassar A, Hussain A., 1985, Mechanism of nasal drug absorption of drug II: absorption of L-tyrosine and the effect of structural modification on its absorption. J Pharm Sci , 1298-1301.
33. Huang CH, Kimura R, Nassar R, Hussain A. 1985, Mechanism of nasal absorption of drugs, physicochemical parameters influencing the rate of in situ nasal absorption of drugs in rats. J Pharm Sci , 608-611.
34. Hussain A, Hamadi S, Kagoshima M, Iseki K, Dittert L. 1991,Does increasing the lipophilicity of peptides enhance their nasal absorption.J Pharm Sci ,80: 1180-1181.
35. Hussain A, Kimura R, Huang CH, Kashihara T. Nasal absorption of naloxone and buprenorphine in rats. Int J Pharm 1984; 233-237.
36. Illum L. 2002, Nasal drug delivery: new developments and strategies. Drug Discov Today , 1184-1189.
37. Illum L. In: Mathiowitz E, Chickering DE, Lehr CM Ed, 1999, Bioadhesive formulations for nasal peptide delivery: Fundamentals, Novel Approaches and Development. Marcel Dekker. New York , 507-539.
38. Illum L., 2000, Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci, 1-18.
39. Inagaki M, Sakakura Y, Itoh H, Ukai K, Miyoshi Y.,1985,Macromolecular permeability of the tight junction of human nasal mucosa. Rhinology, 213-221.
40. Jiang XG, Lu X, Cui JB, Qiu L, Xi NZ. 1997, Studies on octanol-water partition coefficient and nasal drug absorption. Yao Xue Xue Bao , 458-460.
41. Kadam S.S., Mahadik, K.R., Pawar, A.P, Paradkar, A.R., 1993,Transnasal delivery of peptides – a review, The East. Pharm. 47–49.
42. Kao HD, Traboulsi A, Itoh S, Dittert L, Hussain A., 2000, Enhancement of the systemic and CNS specific delivery administration of its water soluble prodrugs of L?dopa by the nasal Pharm Res, 978?984.
43. Kern W, Born J, Schreiber H, Fehm HL., 1999, Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes, 557-563.
44. Kotze A.F., Luessen H.L., Thanou M., Verhoef J.C., de Boer A.G., Junginger H.E., Lehr C.M., 1999, Chitosan and chitosan derivatives as absorption enhancers for peptide drugs across mucosal epithelia, in: Mathiowitz E., Chickering D.E. III, Lehr C.M. (Eds.), Bioadhesive Drug Delivery Systems. Fundamentals, Novel Approaches, and Development, Marcel Dekker, New York, 341–386.
45. Kublik H, Vidgren MT., 1998, Nasal delivery systems and their effect on deposition and absorption. Adv Drug Deliv Rev, 157-177.
46. Kucheryanu VG, Kryzhanovsky GN, Kudrin VS, Yurasov VV,Zhigaltsev IV, Intranasal fibroblast growth factors: 1999, delivery into the brain exerts antiparkinsonian effect in mice, in 26th Int. Symp on Controlled Release of Bioactive Materials (Controlled Release Society Inc., Boston MA,), 643.
47. Kuper CF, Koornstra PJ, Hameleers DM, et al. 1992, The role of nasopharyngeal lymphoid tissue. Immunol Today, 219-224
48. Laing JM, Gober MD, Golembewski EK, Thompson SM, Gyure KA, Yarowsky PJ, Aurelian L. Intranasal administration of the growth-compromised HSV-2 vector DeltaRR prevents kainate-induced seizures and neuronal loss in rats and mice. Mol Ther, 870-881, 2006.
49. Lee VHL., 1988, Enzymatic barriers to peptide and protein nose-brain pathway for psychotropic peptides: evidence absorption. CRC Crit Rev Ther Drug Carrier Syst, 69-97.
50. Madara, J.L., Dharmsathaphrn, K, 1985, Occluding junction structure function relationship in a cultured epithelial monolayer. J. Cell Biol., 2124–2133.
51. Mathiowitz E., Lehr C.M., Chickering D., 1998, Drug Delivery-issues in Fundamentals, Novel Approaches and Development, Marcel Dekker, New York ,507–539
52. McMartin C, Hutchinson LE, Hyde R, Peters GE. 1987, Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J Pharm Sci , 535-540.
53. McMartin, C. et al., 1987, Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J. Pharm. Sci. 76:535–540
54. Mistry A, Stolnik S, Illum L., 2009, Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm ,146-157.
55. Ohwaki T, Ando H, Kakimoto F, et al. 1987, Effects of dose, pH, and osmolarity on nasal absorption of secretin in rats. II: Histological aspects of the nasal mucosa in relation to the absorption variation due to the effects of pH and osmolarity. J Pharm Sci; 695-698.
56. Ohwaki T, Ando H, Watanabe S, Miyake Y. 1985, Effects of dose, pH,and osmolarity on nasal absorption of secretin in rats. J Pharm Sci, 550-552.
57. Pardridge WM. 2010, Preparation of Trojan horse liposomes (THLs) forgene transfer across the blood-brain barrier. Cold Spring Harb Protoc 4:pdb.prot5407,
58. Park H., Robinson J.R. , 1987, Mechanism of mucoadhesion of poly(acrylic acid) hydrogels, Pharm. Res. 457–464.
59. Park H., Robinson J.R., 1985, Physicochemical properties of water insoluble polymers important to mucin/epithelial adhesion, J. Control. Release, 47–57.
60. Perras B, Marshall L, Kohler G, Born J, Fehm HL., 1999, Sleep and endocrine changes after intranasal administration of growth hormonereleasing hormone in young and aged humans. Psychoneuroendocrinology, 743-757.
61. Perras B, Pannenborg H, Marchall L, Pietrowsky R, Born J, Fehm HL., 1999, Beneficial treatment of age-related sleep disturbances with prolonged intranasal vasopressin. J Clin Psychopharmacol, 28-36.
62. Peter M. Som, Joel M.A. Shugar, Margaret S. Brandwein, text book of Anatomy and Physiology
63. Pietrowsky R, Struben C, Molle M, Fehm HL, Born J. 1996, Brain potential changes after intranasal administration vs. intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol Psychiatry, 332-340.
64. Pietrowsky R, Thieman A, Kern W, Fehm HL, Born JA. 1996, A nosebrain pathway for psychotropic peptides: evidence from a brain evoked potential study with cholecystokinin. Psychoneuroendocrinology, 559-572.
65. Ponchel G., Touchard F., Duchene D., Peppas N.A., 1987, Bioadhesive analysis of controlled-release systems: III. Bioadhesive and release behavior of metronidazole-containing poly(acrylic acid)-hydroxypropyl methylcellulose systems, Int. J. Pharm. 65–70.
66. Ponchel G., Touchard F., Duchene D., Peppas N.A., 1987,Bioadhesive analysis of controlled-release systems: I. Fracture and interpenetration analysis in poly(acrylic acid-containing systems, J. Control. Release,129– 141.
67. Pontiroli A, Albertto M, Calderara A, Pajetta E, Pozza G., 1991,Absolute bioavailability of nicotine applied to different Nasal administration of glucagon and human calcitonin to nasal regions. Eur J Clin Pharmacol, 585-588.
68. Quay SC. Successful delivery of apomorphine to the brain following intranasal administration demonstrated in clinical study, PR Newswire, July 18, 2001.
69. Read RC, Naylor SC, Potter CW, et al., 2005, Jennings R. Effective nasal influenza vaccine delivery using chitosan. Vaccine, 4367-4374.
70. Reinhard C, Frey II WH (inventors). 2009 Delivery of polynucleotide agents to the central nervous system. United States Patent 12419999.
71. Sakane T, Akizuki M, Yamashita S, Nadai T, Hashida M, Sezaki H., 1991, The transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J Pharm Pharmacol, 449-451.
72. Shi ZQ, Zhang QZ, Jiang XG., 2005, Enhancement of systemic and CNS delivery of meptazinol hydrochloride by intranasal administration to rats. Yao Xue Xue Bao, 754-757.
73. Singh M, Briones M, O’Hagan DT., 2001, A novel bioadhesive intranasal delivery system for inactivated influenza vaccines. J Control Release, 267-276.
74. Smolnik R, Molle M, Fehm HL, Born J., 1999, Brain potentials and attention after acute subchronic Intranasal administration of ACTH4-10 desacetyl-a-MSH in humans. Neuroendocrinol, 63-72.
75. Soane RJ, Frier M, Perkins AC, Jones NS, Davis SS, Illum L., 1999, Evaluation of the clearance characteristics of bioadhesive systems in humans. Int J Pharm, 55-65.
76. Striebel HW, Pommerening J, Rieger A. Intranasal fentanyl titration for postoperative pain management in an unselected population.Anaesthesia 1993; 48(9): 753-757.
77. Sun BL, Shen FP, Wu QJ, Chi SM, Yang MF, Yuan H, Xie FM, Zhang YB, Chen J, Zhang F. Intranasal delivery of calcitonin gene-related peptide reduces cerebral vasospasm in rats. Front Biosci (Elite Ed) ,1502-1513, 2010.
78. Talegaonkar S, Mishra PR., 2004, Intranasal delivery: An approach to bypass the blood brain barrier. Indian J Pharmacol, 140-147.
79. Tobyn M.J., Johnson J.R., Dettmar P.W., 1996, Factors affecting in vitro gastric mucoadhesion: II. Properties of polymer, Eur. J.Pharm. Biopharm. 56–61.
80. Velin D, Kraehenbuhl JP., 2000, Delivery systems and adjuvants for vaccination against HIV. EXS, 227-237.
81. Yamamoto A, Morita T, Hashida M, Sezaki H. Effect of absorption promoters on the nasal absorption of drugs with upper various molecular weights. Int J Pharm 1993; 91-99.
82. Yuki Y, Byun Y, Fujita M, et al., 2001, Production of a recombinant hybrid molecule of cholera toxin B-subunit and proteolipid-proteinpeptide for the treatment of experimental encephalomyelitis. Biotechnol Bioeng, 62-69.
Received on 15th Jun, 2012 | Published on 10th Jul, 2012
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









