 About Authors:
About Authors:
Mr. Davender. S. Bhandari
Department Of Pharmaceutical Science
Kumarhatti, Solan (H.P)
TECHNOLOGY TRANSFER
“Technology Transfer involves transferring technique & responsibility from development phase to regular production group .Whether transfer takes place between two sites, two companies, a company & third party manufacturer, or from R&D to a pilot plant or commercial facility.”
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1246
The feasibility study plays vital role at receiving site, because all the associated knowledge, information & skill essential for appropriate. process & equipment selection. The steps through technology proceeds are represented as
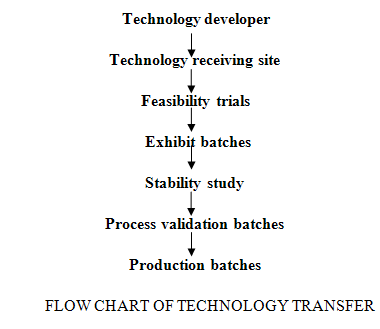
In case of International Technology transfer the technology developer gives rights of product manufacturing to technology receiver. The technology receiver compiles the data satisfactorily and takes feasibility trial for the technology feasibility assessment (Berry, I. R., 2007; pharmapubs1.com).
PROCESS VALIDATION
“Process validation is establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications and quality characteristics.
Benefits of process validation:
- Compliance with government regulation
- Harmonization of global GMP’s
- Internationally acceptable quality is attained
- Reduction in risk of process variables
- Built in quality & hence the small size sample in production batches
- Few batch failure, less work , rejects & wastage
Types of process validation:
(a) Prospective process validation (Premarket validation)
(b) Retrospective process validation
(c) Revalidation
(d) Concurrent Validation
Prospective process validation is done during technology transfer Hence the product under transfer is also undergoes prospective validation. The current project involves international technology transfer of tablets manufacturing process.
[adsense:468x15:2204050025]
OBJECTIVES OF THE STUDY
“To Transfer the technology & optimize the manufacturing process of Metformin Hydrochloride IR tablets for commercial production with successful process validation.”
PLAN OF STUDY
The plan of work designed based on master manufacturing formula
|
· Literature review |
|
· Process flow chart |
|
· Feasibility assessment |
|
· Laboratory trials |
|
· Identification of critical process parameters |
|
· Challenging the identified variables |
|
· Validation protocol |
|
· Execution of validation batches |
|
· Stability study |
|
· Results & discussion |
Drug Profile:
Name of drug: Metformin Hydrochloride
Category: Metformin Hydrochloride is biguainide hypoglycaemic agent used in non insulin dependent diabetes mellitus
Chemical structure:
IUPAC name: N, N-dimethyllimidodicarbonimic diamide hydrochloride
1, 1-dimethylbiguanide hydrochloride
N, N-dimethylbiguanide hydrochloride
N’-dimethylguanylguanidine hydrochloride
Mechanism of action:
Metformin does not cause insulin release from the pancreas and generally does not cause hypoglycaemia, even in large doses. Metformin reduces glucose levels primarily by decreasing hepatic glucose production and by increasing insulin action in muscle and fat.
At a molecular level, these actions are mediated at least in part by activation of the cellular kinase AMP-activated protein kinase (AMP kinase). Metformin also may decrease plasma glucose by reducing the absorption of glucose from the intestine, but this action has not been shown to have clinical relevance
Pharmacokinetics:
Onset of action: Within Day, maximum effect up to 2 weeks
It’s Volume of distribution is 654 ± 358 Lit, protein binding is negligible & not metabolised in liver. The bioavailability of Metformin at fasting stage is 50-60% & elimination half life is 4-9 hrs. Metformin peak time in serum for immediate release is 2-3 hrs & for extended release is 4-8 hrs.It is excreted in urine 90% as unchanged drug. (Davis, S. N., 2006).
Precautions and adverse effects:
Patients with renal impairment should not receive Metformin. Other contraindications include hepatic disease, a past history of lactic acidosis (of any cause), cardiac failure requiring pharmacological therapy, or chronic hypoxic lung disease. The reported incidence of lactic acidosis during Metformin treatment is less than 0.1 cases per 1000 patient-years, and the mortality risk is even lower.
Laboratory tests:
Response to all diabetic therapies should be monitored by periodic measurements of fasting blood glucose and glycosylated haemoglobin levels, with a goal of decreasing these levels toward the normal range (Setter, S.M., 200)
MANUFACTURING STAGES
|
Dispensing of material |
|
Granulation |
|
Drying |
|
Blending & Lubrication |
|
Compression |
|
Coating |
|
Finished Product Analysis |
|
Packaging |
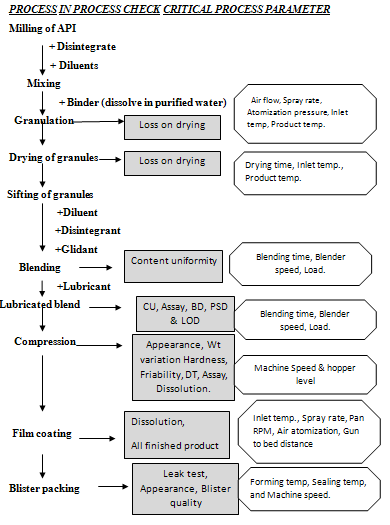
CONCLUSION
Metformin Hydrochloride 500mg, 850mg, 1000mg film coated tablets was prepared using FBE granulation technology on lab scale size. This study was carried out through a systematic plan; critical parameters were optimized to produce a stable & robust manufacturing process.
This project involves international technology transfer, which been transfer within Ranbaxy Lab. Paonta- Sahib. The data provided by technology supplier was studied extensively to understand product behavior trials were taken on lab scale size & also verified feasibility of technology on available sets of facilities and equipments. The critical process variables studied extensively during trial batches.
Three validation batches of commercial scale batch size were taken successfully and monitored the in process critical parameters for commercial batches. Metformin Hydrochloride tablets were prepared within specification for meeting all quality attributes and loaded for stability study. The stability data after three months accelerated stability testing was found to be satisfactory & data matches with previous test results.
The overall successful three consecutive validation batches of Metformin Hydrochloride film coated tablets (500, 850 &1000mg) verifies the international technology transfer success.
REFERENCES
- Agalloco, J., Carleton J.,2008, Validation of Pharmaceutical process. Why Validation?. Informa Healthcare:NY, 3rd edition,3.
- Ahmed, S. U.,Nani, V.,Wadgaonkar D., 2005. Scale up process validation & Technology Transfer, In:Shargel, L., Kanfer, I.(Eds.). Generics Drug Product Development: Solid Oral Dosage form, vol.143, Marcel Dekker, New York, pp. 95-136.
- Bankar, G. S., Anderson, N. R., 1986. Tablets, In: Lachman, L., Liberman, H. A. and Kaniz, J. L(Ed.).The Theory and Practice of Industrial Pharmacy, Varghese Publishing House. Bombay, pp. 293-342,343-348.
- Chao, A. Y., Forebes, F. J., Johnson, R. F., 2003. Prospective Process Validation, In: Nash, R. A., Watcher A. H. (Eds.), Pharmaceutical Process Validation, vol.129, Marcel Dekker, New York/Basel, pp.7-31.
- Manthan, D. J., 2008. Facets of Technology Transfer: A Perspective of Pharmaceutical Industry. Journal of Intellectual property Right .13, 28-34.
- Mehta, R.M., 2002.Processing of Tablets, pharmaceutics-1, Vallabh Prakashan, Delhi, 238-267.
- Patel, J. K. et al., 2009. Aqueous-Based Film Coating of Tablets: study the effect of critical process parameters. Pharma Tech Research.1, 235-240.
- Pfeffer, et al., 2006.Direct Compression Formulation and Process: US Patent 20060210627, 21 Sep.
- Rane, M., Parmar, J.,2009. Tablet Formulation design and Manufacturing: Oral Immediate Release Application. Pharma Times, 41, 21-24.
- Sweetman, S. C.,2007. Metformin Hydrochloride, Sweetman, S. C. (Ed.).The complete drug references, vol. 1, Pharmaceutical Press, London/Chicago, pp.411-412.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









