 About Authors: Arun Dureja*, Kalpana Divekar and M.S.Sandhyavali
About Authors: Arun Dureja*, Kalpana Divekar and M.S.Sandhyavali
Department of Pharmaceutical Chemistry,
Dayananda Sagar College of Pharmacy,
Kumaraswamy Layout,
Bangalore
Abstract
A series of 3-(4-acetyl-5H-5-phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)- 2H-chromen-2-one derivatives were synthesized and evaluated for their anticonvulsant and antioxidant activities. Initially Ethyl-2-oxo-2H-chromene-3-carboxylate was prepared by reacting Salicylaldehyde with Diethyl malonate in presence of piperidine. This compound was treated with hydrazine hydrate to give aromatic hydrazides. Those hydrazides were refluxed with different aldehydes to form arylidines, which were then treated with acetic anhydride to form 3-(4-acetyl-5H-5-phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)- 2H-chromen-2-one derivatives. The structures of synthesized compounds were characterized and confirmed by IR and 1HNMR and then screened for antioxidant activity by DPPH reduction method and anticonvulsant activity by MES method. The investigation revealed that out of eight newly synthesized derivatives five were found to possess significant anticonvulsant activity but none of the derivative was found with antioxidant activity.
[adsense:336x280:8701650588]
Introduction
By far the most numerous and most important heterocyclic systems are those of five and six members.1 Almost all the compounds we know as drugs, vitamins, and many other natural products are heterocycles.
Heterocycles form by far the largest of classical divisions of organic chemistry and are of immense importance biologically and industrially. The majority of pharmaceuticals and biologically active agrochemicals are heterocyclic while countless additives and modifiers used in industrial applications ranging from cosmetics, reprography, information storage and plastic are heterocyclic in nature. One striking structural feature inherent to heterocycles, which continues to be exploited to great advantage by the drug industry, lies in their ability to manifest substituent around a core scaffold in defined three dimensional representations2.
1, 3, 4-oxadiazoles have attracted an interest in medicinal chemistry as ester and amide bioisosteres for a number of biological targets. As a consequence of these characteristics the 1,3,4-oxadiazole derivatives have been found to exhibit diverse biological activities such as antioxidant3, antimicrobial4, anti-HIV5, antitubercular6, anticonvulsant7, anti-inflammatory8, antiviral9, pesticides9 and insecticides9, antihypertensive10 and anthelminthic11.
The widespread use of 1, 3, 4-oxadiazoles as a scaffold in medicinal chemistry establishes this moiety as an important bioactive class of heterocycles. These molecules are also used as pharmacophores due to their favourable metabolic profile and ability to engage in hydrogen bonding. 1, 3, 4-oxadiazoles have proved to be useful in material science as probe for their fluorescence and scintillation properties.
Materials and Methods
All the solvents were of LR grade and were obtained from Merck, CDH and S.D. Fine Chemicals. Melting points were determined in open capillary tubes and are uncorrected. The FT-IR spectra were recorded in KBr pellets on a Shimadzu FT-IR spectrophotometer. 1HNMR spectra were obtained on DMM X-200 MHz. Brookfield using CDCl3 and DMSO. The chemical shifts (δ) are reported in ppm downfield from standard internal reference Tetramethylsilane (TMS). The physiochemical constants of the synthesized compounds are given in Table I.
Step-I:- Synthesis of Ethyl-2-oxo-2H-chromene-3-carboxylate12, 3
Salicylaldehyde 1 (30.5g, 0.50 mole) and diethyl malonate2 (44g, 0.55 mole) were dissolved in 100 ml absolute ethanol. To this mixture 2.5 ml of piperidinewas added as a catalyst and few drops of glacial acetic acid, and the solution was heated under reflux for 3 hours. The product crystallized readily as the solution cooled and was finally stored overnight in a refrigerator. The crystalline product was collected by filtration and washed with a solution made from 95% ethanoland water (4:6). The crude product was dried in the air and recrystallized from ethanolto give white crystals.
IR (KBr, cm-1): 3059 (Ar CH str),1768 (C=O, lactone), 1608 (C=O, ester), 1201 (C-O, coumarin), 783 (Ar CH bend).1H NMR (δ ppm ): 1.42 (3H, t, CH3), 4.44 (2H, q, CH2), 7.27-7.69 (4H, m, Ar-H), 8.53 (1H, s, Ar-H).
Step II:- Synthesis of 2-Oxo-2H-chromene-3-carbohydrazide13, 4
Compound 3 (2.18g, 0.1 mol) and hydrazine hydrate 98% (1.0g, 0.2 mol) were refluxed in 50ml of 95% ethanol for 6 hours. The resultant mixture was concentrated, cooled and poured onto crushed ice. The solid mass thus separated out was filtered, dried and purified by recrystallization from ethanol. IR (KBr, cm-1): 3381 (NH2), 3286 (NH str), 3037(Ar CH str),1691 (C=O, lactone), 1616 (C=O, ketone), 1196 (C-O, coumarin) and 750 (Ar CH bend).
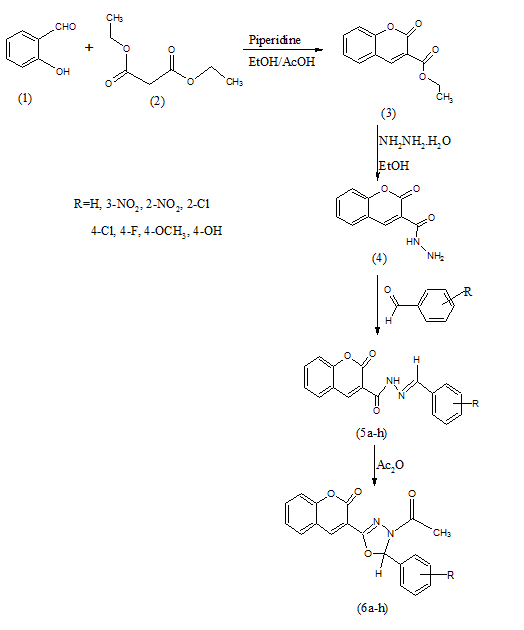
Step III: Synthesis of 2-Oxo-N-[(1E)-1-phenylethylidene]-2H-chromene-3- carbohydrazide14, 5a-h
A mixture of 4 and benzaldehyde were dissolved in ethanol and refluxed on water bath for 5-6 hours in presence of few drops of acetic acid. The reaction mixture was poured into ice-cold water and solid was filtered out. The dried solid was recrystallized from ethanol. IR (KBr, cm-1): 3055 (Ar CH str),1701(C=O, lactone), 1614 (C=O, ketone), 1527 (C=N), 1205 (C-O, coumarin) and 748 (Ar CH bend).Similarly, other compounds of the series were synthesized.
Step IV: Synthesis of 3-(4-acetyl-5H-5-phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)- 2H-chromen-2-one15, 6a-h
A mixture of 2-oxo-N-[(1E)-1-phenylethylidene]-2H-chromene-3-carbohydrazide5 (0.002 mol) and excess of acetic anhydride (10 mL) was refluxed for 3 hours. The excess acetic anhydride was distilled off at reduced pressure and residue was poured into ice cool water. The solid product was filtered and recrystallized from ethanol to give 3-(4-acetyl-5-methyl-5-phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)-2H-chromen-2-one.IR (KBr, cm-1): 3055 (Ar CH str),1681 (C=O, lactone), 1618 (C=O, ketone), 1572 (C=N), 1267 (C-O-C) [oxadiazole nucleus], 1195 (C-O, coumarin), 752 (Ar CH bend). 1H NMR (δ ppm ): 2.38 (3H, s, COCH3), 7.32-7.53 (4H, m, Ar-H), 7.8 (2H, d, Ar-H), 8.11 (2H, d, Ar-H), 8.6 (1H, s, Ar-H), 8.7 (1H, s, CH of oxadiazole).
Similarly, other compounds were synthesized from 5a-h.
Table I: Physiochemical properties of synthesized 1,3,4-oxadiazole derivatives
|
Compound |
R |
Molecular formula |
Mol. wt |
% yield |
M.P (0C) |
|
6a |
H |
C19H14N2O4 |
334 |
58 |
176 |
|
6b |
3-NO2 |
C19H13N3O6 |
379 |
60 |
189 |
|
6c |
2-NO2 |
C19H13N3O6 |
379 |
66 |
190 |
|
6d |
2-Cl |
C19H13N2O4Cl |
368 |
62 |
185 |
|
6e |
4-Cl |
C19H13N2O4Cl |
368 |
80 |
186 |
|
6f |
4-F |
C19H13N2O4F |
352 |
57 |
202 |
|
6g |
4-OCH3 |
C20H16N2O5 |
364 |
70 |
165 |
|
6h |
4-OH |
C19H14N2O5 |
350 |
74 |
172 |
Pharmacological activity
Antioxidant Activity16:
In the present study DPPH scavenging method was used for screening the antioxidant activity. The antioxidant activity of the synthesized compounds was expressed as IC50 values. Ascorbic acid was used as a standard drug.
DPPH is stable free radical that can accept an electron or hydrogen radical and must thus be converted to a stable, diamagnetic molecule. DPPH has an odd electron and so has a strong absorption band at 517 nm when this electron becomes paired off, the absorption decreases stoichiometrically with respect to the number of electrons or hydrogen atoms taken up, such change in the absorbance by this reaction has been extensively adopted to test the capacity of several molecules to act as free radical scavengers. Various concentrations of the test compound in DMSO were added to a 5.0 ml (0.2mM) solution of DPPH radical in methanol [9]. The mixture was shaken vigorously andallowed to stand for 30 min; the absorbance of the resulting solution at 517 nm was measured using shimadzu UV spectrophotometer. None of the Compound was found with antioxidant activitywhen compared with the standard drug. The values are summarized as IC50. (Table II).
Anticonvulsant activity of 1, 3, 4-oxadiazole derivatives17:
The study of anticonvulsant activity of 1, 3, 4-oxadiazole derivatives was done by maximal electro-shock-induced convulsions in rats.The MES-convulsions are divided into five phases such as (a) tonic flexion, (b) tonic extensor, (c) clonic convulsions, (d) stupor and, (e) recovery or death. The abolition of extensor (tonic phase) in drug treated group was taken as criteria for anticonvulsant activity.
Animals were divided into ten groups each consisting of six rats. One group was used as control, one group for Phenytoin and other groups were used for test compounds. A suspension of the test compound was made in 1% Carboxy Methyl Cellulose (CMC) solution so as to get the dose of 20mg/ml. The normal response of the test animals in the control group was recorded by placing ear electrode on the ears of the animal and an electric current of 150 mA was applied for 0.2 sec.
Phenytoin was injected intraperitoneally to the standard group. At the end of 30 minutes, the animals were subjected to electro convulsions. The test compounds were administered in the similar manner to the other groups by oral route. At the end of 1 hour, the animals were subjected to electro convulsions. The time for different responses was noted. The readings are tabulated in table No III.
Results and Discussion
The antioxidant activity was carried out by DPPH method. The activity screening suggests that none of the derivative showed any activity by DPPH reduction method.All the test compounds were screened for anticonvulsant activity by maximal electro-shock induced convulsions using ear electrode in Wistar Albino rats. The Phenytoin was used as positive control of dose 25mg/kg and normal saline as solvent control. Out of 8 derivatives, 3-nitro, 4-chloro, 4-fluoro, 4-methoxy and 4-hydroxy derivatives were found to show significant activity with p<0.001 value, 2-chloro derivative was found to show moderate activity with p<0.01, phenyl derivative was evaluated as mildly active with p<0.05 and other two compounds were found non significant with p>0.05.
Table No.II: Antioxidant activity of synthesized compounds by DPPH method
|
Sl.No |
Compound |
% Inhibition |
IC50 ± SEM DPPH Method |
|
1. |
Compound 6a |
19.97 – 85.95 |
47.47 ± 2.473 |
|
2. |
Compound 6b |
3.07-64.92 |
197.96 ± 2.454 |
|
3. |
Compound 6c |
7.4 - 48.75 |
>500 |
|
4. |
Compound 6d |
13.87 - 77.45 |
60.93 ± 1.560 |
|
5. |
Compound 6e |
12.60 - 85.95 |
>500 |
|
6. |
Compound 6f |
14.70 - 69.70 |
130.8 ± 3.602 |
|
7. |
Compound 6g |
4.9 - 74.77 |
90.26 ± 2.442 |
|
8. |
Compound 6h |
6.85 – 69.42 |
91.70 ± 2.778 |
|
STD. |
Ascorbic acid |
44.95 - 95.5 |
12.7 ± 0.68 |
Table III: Effect of 1, 3, 4-oxadiazole derivatives by maximal electric shock (MES) induced convulsions in rats.
|
SL. No. |
Compounds |
Body weight |
Dose (mg/kg) |
Extensor phase (sec) (Mean±SEM) |
|
1. |
Control |
115 |
Saline Solution 1ml |
13.0 ±0.577 |
|
2. |
Phenytoin |
110 |
25 |
0.667 ± 0.333*** |
|
3. |
6a |
120 |
200 |
10.166±0.307* |
|
4. |
6b |
117 |
200 |
8.1±0.737*** |
|
5. |
6c |
110 |
200 |
11.33±0.494ns |
|
6. |
6d |
115 |
200 |
9.5±0.428** |
|
7. |
6e |
115 |
200 |
5.5±0.619*** |
|
8. |
6f |
116 |
200 |
7.833±0.307*** |
|
9. |
6g |
117 |
200 |
4.333±0.333*** |
|
10. |
6h |
120 |
200 |
6.834±0.792*** |
Values are expressed as Mean ± SEM, [number of animal (n) = 6]
Values were analysed one way ANOVA followed by Tukey-Kramer’s test.
Where, * represents mild significant at P<0.05, ** represents moderate significant at P<0.01, *** represents highly significant at P<0.001, ns represents non significant at P>0.05 Vs control.
References
1. Gupta R. R, Kumar M, Gupta B. Heterochemistry, Springer Verlog Berlin Heidelberg. 1999 (2): 8-11.
2. Dabiri M, Salehi P, Baghbanzadeh M, Bahramnejad M. Tetrahedron Letters. 2006; 47: 6983-86.
3. M Vijey Aanandhi, Mohammed Hashim Mansoori, S Shanmugapriya, Shiny George and P Shanmugasundaram. Synthesis and In- vitro antioxidant activity of substituted Pyridinyl- 1, 3, 4- oxadiazole derivatives. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2010; 1(4): 1083-86.
4. Niti Bhardwaj, S. K. Saraf, Pankaj Sharma and Pradeep Kumar. Synthesis, Evaluation and Characterization of Some 1, 3, 4-Oxadiazoles as Antimicrobial Agents. E-Journal of Chemistry. 2009; 6(4): 1133-38.
5. Kucukguzel S.G, Oruc E.E, Rollas S, Sahin F and Ozbek A. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002; 37(3): 197-206.
6. Preethi R Kagthara, Niraj S Shah, Rajeev K Doshi and Parekh H.H. Synthesis of 2,5-disubstituted-1,3,4-oxadiazoles as biologically active heterocycles. Indian J.Chem. 1999; 38B: 572-76.
7. Narayan Deshpande, Rao Y V, Kandlikar R P, Devender A Rao & Reddy V M. A study of anticonvulsant activity with 6,8-dibromo 3-(5- aryl-1,3,4-oxadlazol-2yl)-methyl)-2-methyl-4(3h) quinazolinones. Indian J. Pharmac.1986; 78: 127-28.
8. Mohd Amir, S A Javed and Harish Kumar. Synthesis of some 1,3,4-oxadiazole derivatives as potential Anti-inflammatory agents. Indian J. Chem. 2007; 46B: 1014-19.
9. N Vasanth Kumar and Uday C Mashelkar. Synthesis of 1, 2, 4-oxadiazole and biological activities such as analgesics, anti-inflammatory, antimicrobial, antiviral pesticides and insecticides. Indian J .chem. 2007; 46B:216-20.
10. Manish Kumar Mishra, Gupta A K, Negi S and Meenakshi Bhatt. Synthesis of some new oxadiazole with Antimicrobial activity. International Journal of Pharma Sciences and Research. 2010; 1(3): 172-77.
11. Kalpesh Patel, Jayachandran E, Ravishsh, Vijaya Javali and Sreenivasa G.M. Synthesis, characterization and Anthelmintic activity of new oxadiazole incorporated with imidazole and pyrazole. International Journal of Pharma and Bio Sciences. 2010; 1 (3): 1-13.
12. Horning E. C, Horning M. G, and Dimmig D. A. 3-CARBETHOXY COUMARIN. Organic Syntheses, 1955; 3: 165.
13. Pattan Shashikant R, Rabara P A, Pattan Jayashri S, Bukitagar A A, Wakale V S and Musumade D S. Synthesis of some novel substituted 1,3,4-oxadiazole and pyrazole derivatives for pyrazole derivatives for antitubercular activity. Indian J. Chem. 2009; 48:1453-56.
14. Siddappa K, Mallikarjun K, Tukaram Reddy, Mallikarjun M, Reddy C.V and Mahesh Tambe. Synthesis, Characterization and Antimicrobial Studies of N1-[(1E)-1-(2-Hydroxyphenyl) ethylidene]-2-oxo-2H-chromene-3-carbohydrazide and its Metal Complexes. E-Journal of Chemistry. 2009; 6(3): 615-24.
15. Mashooq A Bhat, Nadeem Siddiqui and Suroor A Khan, Synthesis of novel 3-(4- acetyl-5H/ methyl-5-substituted phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)-2H-chromen-2-ones as potential Anticonvulsant agents. Acta Poloniae Pharmaceutica- Drug Research. 2008; 65: 235-39.
16. Shirwaikar Annie, HN Aswatha Ram and Mohpatra P. Antioxidant and Antiulcer activity of aqueous extract of a polyherbal formulation. Indian J of Experimental biology. 2006; 44 (6): 474-80.
17. Tandon V R, Gupta R K. An experiment evaluation of anticonvulsant action of Vitex-Negundo. Indian J. physiol. Pharmacol. 2005; 49(2): 199-205.
Reference ID: PHARMATUTOR-ART-1019
FIND OUT MORE ARTICLES AT OUR DATABASE









