About Authors:
KALPESH PATEL, JAYACHANDRAN E.*, SREENIVASA G.M.
* P.G. Department of Pharmaceutical Chemistry,
S.C.S. College of Pharmacy, Harapanahalli-583131,
Karanataka, India.
*drjc_2006@rediffmail.com, kalpesh.m.pharm@gmail.com
Abstract
Various substituted 3-amino-1-(2,4-dinitro phenyl)-5-[(5-substituted-1,3,4-oxadiazol-2-yl)amino]-1-H-pyrazole-4-carboxyamide and (5E)-5-[4-(dimethylamino) benzylidene]-3-(5-substituted-1,3,4-oxadiazol-2-yl)-2-phenyl-3,5-di hydro-4H-imidazol-4-one have been synthesised and evaluated for antiinflammatory activity. Structure of these products has been established by IR, 1H NMR data. Significant activity were observed for some members of the series.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1276
INTRODUCTION
Oxadiazole are cyclic compounds containing one oxygen and two nitrogen atom in a five membered ring. The sequence of these atoms may be different as 1,2,4-oxadiazole(a), 1,2,5-oxadiazole(b), 1,2,3-oxadiazole(c) and 1,3,4-oxadiazole(d).
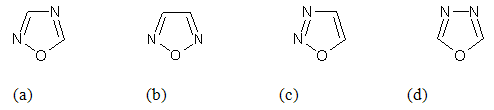
1,3,4-oxadiazoles have been known for about eighty years and investigations in field have been intensified due to the large number of uses and applications in most diverse area. Moreover, ring cleavage reactions of 1,3,4-oxadiazoles have also accelerated interest in various field like medicinal chemistry, polymer industry etc.
The oxadiazole drugs were the first effective chemotherapeutic agents to be employed systematically for the prevention and cure of bacterial infection in human beings.Oxadiazole derivatives are well known to have number of biological activities like Analgesic1, Anthelmintic1, antimicrobial1, Antiinflammatory2-5, Antitubercular6, Anticonvulsant7, Diuretic, CNS depressant, Antiviral, Herbicidal8, Insecticide8, Anticancer9, Antipyretic, Antimitotic, Antiemetic activities.
Imidazole was Planar, Five membered heteroaromatic molecule having two nitrogen. Named first as gluoxaline (first synthesis with glyoxal and ammonia). Amphoteric nature, susceptible to electrophilic and nucleophilic attack. High stability to thermal, acid, base, oxidation and reduction conditions. Extensive intramolecular hydrogen bonding. The reduction products, named as are other rings of five atoms, are imidazoline and imidazolidine and their derivatives structure given below,
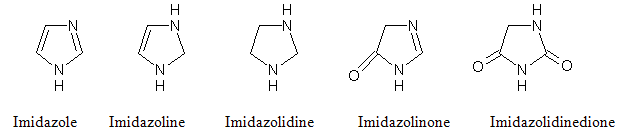
Imidazole exhibit diverse biological activities like Antimicrobial10, Analgesic11, Antiinflammatory12-14, Anthelmintic, Anti-HIV, Treatment of hypoxic tumour cells, CNS depressants15, Antiviral, Antitubercular16,Anticancer17, Antihistamine.
Pyrazole is the name given by “LUDWIG KNORR” to this class of compounds in 1883. The simple doubly unsaturated compound containing two nitrogen and three carbon atoms in the ring, with the nitrogen atoms neighboring, is known as pyrazole. The reduction products, named as are other rings of five atoms, are pyrazoline and pyrazolidine.
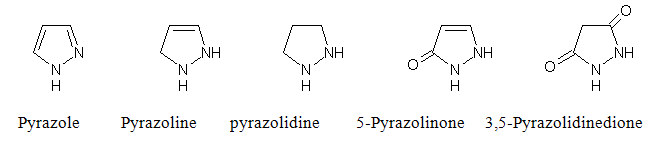
Several pyrazoline substitution products are used in medicine as Anti-inflammatory18-19 Analgesic, Antioxidant21, Antipyretic, Diuretic, Bovine anaplasmosis, Treatment of rheumatic disorders, Antimicrobial20, Antidepressant, Anticonvulsant, Anticancer20, Antiparasitic activity, Antimalerial, Antimycotics, Antidiabetic.
[adsense:468x15:2204050025]
MATERIAL AND METHODS
Bovine serum albumin (Loba Chem) diclofenac sodium (standard) and all other chemicals were of analytical grade.
Melting point was determined by open capillary tube method and are uncorrected. T.L.C. was run on silica gel G plates using ethyl acetate:pet.ether (10:1) as developing solvent for the purity of the compounds. I.R. Spectra were recorded on SHIMADZU FTIR-8400S spectrophotometer by using KBr pellets technique.
SCHEME -I
1st Step :
Synthesis of 2-(Substituted methylidene) hydrazine carboxamide (aldehyde semicarbazone) :
Take 0.1 mol of semicarbazide HCl and 0.1mol of different aromatic aldehydes, sodium acetate as catalyst and stirr for 3 hrs with glass rod and added alcohol then heated on hot plate to concentrate it . Add little alcohol if mixture become solid then heat on a hot plate until dissolve with clicking sound. Kept it over night. Then added alcohol (50ml) & heated on a hot plate, cool at room temperature. Add cold water and filter the product under the vacuum & dry.
2nd Step :
General synthesis of NaOI solution:
NaOI solution prepared by dissolving 8.6 g Sodium Carbonate , 17 g Iodine and 15 g potassium Iodide in Distilled Water.
Synthesis of 5-substituted-1,3,4-oxadiazol-2-amine :
Into aqueous suspension of 4.2 g of 2-(Substituted methylidene) hydrazine carboxamide (aldehyde semicarbazone) add 100 ml of NaOI solution . The combination mixture was heated at 80-85 0c with continuous stirring for 3 hours. Allow stand and cool at room temperature. The precipitated 5-substituted-1,3,4-oxadiazol-2-amine was collected by suction filter. Washed with cold water and dried. The product was recrystalised from ethanol.
3rd Step :
General synthesis of bis-s-methyl ethylene cyanoacetamide :
Synthesis of cyanoacetamide :
In a 1 litre glass stopped conical flask 50ml of concentrated ammonia was taken, 60ml of ethylcyanoacetate (1.77m) is added drop by drop in ice cold condition. After the complete addition the solution is allowed to stand for 2hrs. in ice cold condition. During this period a white needle shaped crystals were precipitated and are filtered, washed with cold solution of ethanol.
bis-s-methyl ethylene cyanoacetamide :
To a solution of 13.2g of KOH in a minimum quantity of water, cooled in ice bath, added 30ml dimethyl formamide (DMF) and 0.1mol of cyanoacetamide (8.4g). The mixture was treated drop wise with cooling and stirring, 0.1mol of carbondisulphide was added drop wise below 00C and stirred for an hour, then the reaction mixture was allowed to stand for one hour at room temperature then 20ml of dimethylsulphate was added drop by drop with stirring at 10 to 150C. The mixture was allowed to stand for 12hrs. at room temperature and was poured on to ice water. The solid obtained was filtered and washed with water to get bis-s-methyl ethylene cyanoacetamide.
Synthesis of (2Z)-2-cyano-3-(methylsulfanyl)-3-[(5-substituted-1,3,4-oxadiazol-2-yl) amino]prop-2-enamide :
A mixture of 5-substituted-1,3,4-oxadiazol-2-amine (0.1 mol)and bis-s-methyl ethylene cyanoacetamide (0.1 mol) were refluxed in ethanol for 2 hrs. and later excess of ethanol was distilled off and poured onto crushed ice. The product obtained was filtered and recrystallised from ethanol.
4th Step :
Synthesis of 3-amino-1-(2,4-dinitro phenyl)-5-[(5-substituted-1,3,4-oxadiazol-2yl) amino]-1-H-pyrazole-4-carboxyamide :
A mixture of (2Z)-2-cyano-3-(methylsulfanyl)-3-[(5-substituted-1,3,4-oxadiazol-2-yl) amino]prop-2-enamide (0.1 mol) and 2,4-dinitro phenyl hydrazine HCl (0.1 mol) in equimolar concentration wererefluxed in ethanol for 2 hrs. and later excess of ethanol was distilled off and poured onto crushed ice. The product obtained was filtered and recrystallised from mixture of ethanol and DMF.

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SCHEME –II
3rd Step :
Preparation of oxazolone : (2-phenyl-4-p-dimethylaminobenzylidenyl-5-oxazolone)
A mixture of 37.3 g (26ml, 0.25 mol) of N N`-dimethyl aminobenzaldehyde, 45g (0.25mol) of benzoyl glycine (hippuric acid), 77g (71.5ml, 0.75 mol) of dry acetic acid and 20.5g (0.25mol) of anhydrous sodium acetate was placed in 500ml conical flask and heated on electric hot plate with constant shaking. As soon as the mixture has liquefied completely, the flask was transferred to water bath and refluxed for 2 hrs. 100ml of ethanol was added slowly to the contents of the flask. The mixture was allowed to stand overnight. The crystalline product obtained was filtered by suction. It was washed with two 25ml portions of boiling water and recrystallisation from benzene dried at 100oC. the dry material melted at 167-168oC.
4th Step :
Synthesis of (5Z)-5-[4-(dimethylamino)benzylidene]-3-(5-substituted -1,3,4-oxadiazol -2-yl)-2-phenyl-3,5-dihydro-4H-imidazol-4-one :
5-substituted-1,3,4-oxadiazol-2-amine (0.01 mol) and 4-[4-(dimethylamino) benzylidene]-2-phenyl-1,3-oxazol-5(4H)-one (oxazolone) (0.01 mol) was refluxed in pyridine for 6-8 hrs. The excess of pyridine was distilled off and resulting mass was poured into crushed ice. Filter and recrystalised using mixture of ethanol and DMF.
The denaturation of proteins as one of the causes of inflammation is well documented22-24. Production of auto-antigens in certain rheumatic diseases may be due to in vivo denaturation of proteins. A number of antiinflammatory drugs are known to inhibit the denaturation of proteins. Muzushima25 and other have employed protein denaturation as an in vitro screening model for antiinflammatory compounds. Bovine serum albumin (Loba Chem) diclofinac sodium (standard) and all other chemicals of analytical grade.
Inhibition of albumin denaturation was studied according to Muzushima and Kabayashi with significant modification. The test compounds were dissolved in minimum amount of dimethyl formamide (DMF) and diluted with phosphate buffer (0.2 M Ph 4 - 7.4). Final concentration of DMF in all solutions was less than 2.5%. The test solution (1 ml) containing different concentrations of drug was mixed with 1mM albumin (1 ml) in phosphate buffer and incubated at 270±10C in incubator for 15 min. Denaturation was induced by keeping the reaction mixture at 600±10C in water bath for 10 min. After cooling the turbidity was measured at 660 nm. Percentage of inhibition of denaturation was calculated from control where no drug was added. Each experiment was done in triplicate and average was taken26( Table no. 4).

RESULTS AND DISCUSSION
Anti-inflammatory activity (in-vitro):
Synthesis and pharmacological screening of 3-amino-1-(2,4-dinitro phenyl)-5-[(5-substituted-1,3,4-oxadiazol-2yl)amino]-1-H-pyrazole-4-carboxyamide and (5E)-5-[4-(dimethylamino)benzylidene]-3-(5-substituted-1,3,4-oxadiazol-2-yl)-2-phenyl-3,5-di hydro-4H-imidazol-4-one were tested for the antiinflammatory activity by using inhibition of albumin denaturation technique compared to standard diclofinac sodium significant antiinflammatory activity.
Among compounds tested P1 (50%), P2 (46.87%), P4 (69.79%), P6 (54.86%), P8 (46.87%), I1 (63.10%), I4 (69.82%), I8 (50.01%) showed significant antiinflammatory activity compare to standard Diclofenac (95.52%).The tested compounds P1, P2, P4, P6, P8, I1, I4, I8 showed significant antiinflammatory activity (in vitro) at low and high concentration compared to standard.
REFERENCES
1) H.M.Vagdevi and V.P.Vaidya, Synthesis of 2-(2`-aryl-3`-acetyl -1`,3`,4`-oxadiazolyl) aminonaphtho [2,1-b] furans and their biological activities. Indian Journal of Heterocyclic Chemistry,10: 253-260,(2001).
2) Asif Husain, et al., Synthesis and biological evaluation of 2-[3-(4-methoxy phenyl)propan -3-one ]-5-(substituted phenyl)-1,3,4-oxadiazoles. Indian Journal of Heterocyclic Chemistry, 17: 265-266, (2008).
3) Harish Rajak,Murli Dhar Kharya and Pradeep Mishra, Synthesis of Some Novel Oxadiazole and Oxadiazoline Analogues for Their Antiinflammatory Activity. Yakugaku Zasshi The pharmaceutical Society of Japan, 127 (10) : 1757-1764,(2007).
4) V.N.Sonar, Shaik Khadar Yazadan and N. Sreenivasulu, Synthesis of oxadiazolylindole derivatives and their antiinflammatory activity. Indian Journal of Heterocyclic Chemistry, 10: 299-302,(2001).
5) M.S.Y Khan, Gita Chawla and M Asad Mueed, Synthesis and biological activity of some isoniazid based 1,3,4-oxadiazole derivatives. Indian Journal of Chemistry, 43B: 1302-1305, (2004).
6) S.R.Dhol, et al., Synthesised of certain 1,3,4-oxadiazoles as potential Antitubercular and antimicrobial agents. Indian Journal of Heterocyclic Chemistry, 15: 63-64, (2005).
7) Khan MSY, Khan RM, Sushmadrabhu, Synthesis and anticonvulsant and antimicrobial activity of some new 1,3,4-oxadiazole derivatives. Indian Journal of Heterocyclic Chemistry, 11: 119-122, (2001).
8) Mohammed A. E., et al., Synthesis and biological activities of some 1,3,4-oxadiazoles and bis-(1,3,4-oxadiazoles). Journal of Islamic Academy of sciences,(4:3):184-191, (1991).
9) Pinaki Sengupta & et al., Evaluation of anticancer activity of some 1,3,4-oxadiazole derivatives. Indian Journal of Chemistry, 47B: 460-462, (2008).
10) Sangeet Rajpurohit, et al., Synthesis of 5`-aryl substituted –(4`-benzylidene-4,5-dihydro-5-oxo-1-(H)-imidazolo)-1`, 3`, 4`-oxadiazoles and their antimicrobial activity. Indian Journal of Heterocyclic Chemistry, 15: 129-132, (2005).
11) Sushma Drabu, et al., Synthesis and pharmacological activity of some 2-substituted 4,5- diphenyl Imidazoles. Indian Journal of Heterocyclic Chemistry, 16: 63-64, (2006).
12) Rajiv Dahiya, et al., Synthesis and biological activity of peptide derivatives of iodoquinazolinones/nitroimidazoles. Molecules, 13: 958-976, (2008).
13) Sushma Drabu, Nitin Kumar and Siddeswaran Munirajan, Synthesis and antiinflammatory activity of some 3-substituted indolo[2,3] imidazoles. Indian Journal of Heterocyclic Chemistry, 15: 91-92, (2005).
14) Priya V Frank, K S Girish, Balakrishna Kalluraya, Solvent free microwave assisted synthesis of oxadiazoles containing Imidazole moiety. J.Chem. Sci., 119(1): 41-46, (2007).
15) Farzin Hadizadeh, et al., Synthesis and antidepressant activity of N- substituted Imidazole-5-carboxamides in forced swimming test model. Iranian Journal of Pharmaceutical Research, 7: 29-33, (2008).
16) Amini M., et al., Synthesis and Antitubercular activity of of new N,N -diaryl-4-(dichoroimidazole-2-yl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxamides. DARU, 16(1): 9-12, (2008).
17) Asolankee K. Kapadia, et al., Synthesis and anticancer activity of pyrazoline substituted benzylidene imidazolinones. Oriental Journal of chemistry, 17(2): 315-316, (2001).
18) Hiremath SP, et al., Synthesis and biological activities of new 5-hydrazino-10-substituted-7H-indolo[2,3-c]isoquinolines and 1-(10-substituted-7H-indolo[2,3-c]isoquinolin-5-yl)-3,5-disbstituted pyrazoles,-3 methyl pyrazol-5-ones –3,5-disubstituted pyrazoles. Indian Journal of Chemistry, 41B: 394-399, (2002).
19) V.H.Bhaskar, B.R.Balakrishnan and B. Sangameswaran, Synthesis of 4,5-disubstituted -3-methyl -1,3a,4,5-tetrahydropyrazolo[3,4-c] pyrazoles and their antiinflammatory activity. Indian Journal of Heterocyclic Chemistry, 17: 131-134, (2007).
20) Nimavat KS, Popat KH, Joshi HS., Synthesis, anticancer, antitubercular and antimicrobial activity of 1-substituted-3aryl-5-(3'-bromophenyl)-pyrazolines. Indian Journal of Heterocyclic Chemistry, 12: 225-228, (2003).
21) Anand Kumar Tengli, Shrishailappa Badami, B.R.Prashantha Kumar et al., Microwave assisted synthesis of pyrazoline derivatives and their antiangiogenic and antioxidant activities. Indian Journal of Heterocyclic Chemistry, 16: 333-336, (2007).
22) W.G. Spector and D.A. Willough, Nature, 196, 1104(1962).
23) K.Ishizaka, Immunological Diseases, Little Brown & Co., Boston, p. 131(1965).
24) F.L.Opie, J. Exp. Med., 117, 425 (1963).
25) Y.Muzushima and M. Kobayashi, Indian J. Pharm. Phamacol., 20, 169 (1968).
26) Gellias and M.N.A. Rao, Indian J. Exp. Biol., 26, 540 (1962).
TABLE NO. 1
ANALYTICAL DATA
|
Sl. No |
Compound Code |
M.P/ B.P°C |
% Yield |
MOL. FORM |
M.Wt. |
C% |
H% |
N% |
|
1 |
P1 |
210-212 |
80% |
C18H12ClN9O6 |
485.79 |
44.50 |
2.49 |
25.95 |
|
2 |
P2 |
240-242 |
82% |
C18H13N9O6 |
451.35 |
47.90 |
2.90 |
27.93 |
|
3 |
P3 |
223-225 |
65% |
C20H18N10O6 |
494.42 |
48.58 |
3.67 |
28.33 |
|
4 |
P4 |
247-248 |
72% |
C18H12N10O8 |
496.35 |
43.56 |
2.44 |
28.22 |
|
5 |
P5 |
242-243 |
74% |
C18H12N10O8 |
496.35 |
43.56 |
2.44 |
28.22 |
|
6 |
P6 |
256-257 |
73% |
C19H15N9O7 |
481.37 |
47.41 |
3.14 |
26.19 |
|
7 |
P7 |
193-195 |
76% |
C16H11N9O7 |
441.31 |
43.55 |
2.51 |
28.56 |
|
8 |
P8 |
249-251 |
75% |
C18H13N9O7 |
467.37 |
46.26 |
2.80 |
26.97 |
|
9 |
I1 |
216-218 |
79% |
C26H20ClN5O2 |
469.47 |
66.45 |
4.29 |
14.90 |
|
10 |
I2 |
200-205 |
83% |
C26H21N5O2 |
435.47 |
71.71 |
4.86 |
16.08 |
|
11 |
I3 |
185-188 |
77% |
C28H26N6O2 |
478.54 |
70.28 |
5.48 |
17.56 |
|
12 |
I4 |
195-197 |
85% |
C26H20N6O4 |
480.47 |
64.99 |
4.20 |
17.49 |
|
13 |
I5 |
223-225 |
86% |
C26H20N6O4 |
480.47 |
64.99 |
4.20 |
17.49 |
|
14 |
I6 |
215-216 |
78% |
C27H23N5O3 |
465.50 |
69.66 |
4.98 |
15.04 |
|
15 |
I7 |
226-230 |
80% |
C24H19N5O3 |
425.43 |
67.76 |
4.50 |
16.46 |
|
16 |
I8 |
218-221 |
78% |
C26H21N5O3 |
451.47 |
69.17 |
4.69 |
15.51 |
Characteristics IR absorption bands of different synthesised compounds are tabulated below:
TABLE NO. 2
|
Sl. No. |
Compound code |
-CONH2 cm-1 |
R-NH2(SYM) cm-1 |
Sec.ARamine cm-1 |
-NO2 cm-1 |
Ar C=C cm-1 |
Ar C-H (Bending) cm-1 |
Cyclic C=N cm-1 |
Cyclic C-O-C cm-1 |
|
1 |
P1 |
3155 |
3284 |
1336 |
1507 |
1466 |
828 |
1612 |
1133 |
|
2 |
P2 |
3101 |
3283 |
1330 |
1507 |
1416 |
830 |
1604 |
1129 |
|
3 |
P4 |
3109 |
3286 |
1348 |
1521 |
1427 |
835 |
1599 |
1133 |
|
4 |
P5 |
3095 |
3281 |
1333 |
1512 |
1426 |
814 |
1585 |
1137 |
|
5 |
P6 |
3072 |
3267 |
1312 |
1504 |
1414 |
830 |
1614 |
1169 |
|
6 |
P7 |
3100 |
3362 |
1333 |
1515 |
1481 |
743 |
1613 |
1147 |
|
7 |
P8 |
3097 |
3272 |
1307 |
1512 |
1421 |
830 |
1620 |
1138 |
|
Sl. No. |
Compound code |
-CONR1R2 cm-1 |
Ter.ARamine cm-1 |
-NO2 cm-1 |
Ar C=C cm-1 |
Ar C-H (Bending) cm-1 |
Cyclic C=N cm-1 |
Cyclic C-O-C cm-1 |
|
8 |
I3 |
1645 |
1369 |
- |
1447 |
824 |
1602 |
1162 |
|
9 |
I4 |
1646 |
1373 |
1530 |
1448 |
853 |
1606 |
1164 |
|
10 |
I5 |
1645 |
1371 |
1529 |
1480 |
851 |
1604 |
1159 |
|
11 |
I6 |
1645 |
1372 |
- |
1449 |
813 |
1601 |
1159 |
|
12 |
I7 |
1645 |
1372 |
- |
1446 |
852 |
1604 |
1159 |
|
13 |
I8 |
1646 |
1372 |
- |
1447 |
852 |
1604 |
1159 |
TABLE NO. 3
NMR Spectral Data
|
Sl. No. |
Compound Code |
Hydrogen |
d(ppm) |
Multiplity |
Solvent |
|
1 |
P4 |
Ar-H Ar-NH2 |
7.28 - 9.12 4.03 |
Multiplet Singlet |
CDCl3 |
|
2 |
P5 |
Ar-H Ar-NH2 |
7.28 -- |
Singlet -- |
CDCl3 |
|
3 |
P6 |
Ar-H Ar-NH2 -OCH3 |
6.99 – 8.08 4.03 3.88 |
Multiplet Singlet Singlet |
CDCl3 |
|
4 |
I3 |
Ar-H -CH3 |
6.70 – 7.71 2.17 |
Multiplet Singlet |
CDCl3 |
|
5 |
I5 |
Ar-H -CH3 |
6.72 – 8.15 2.17 |
Multiplet Singlet |
CDCl3 |
|
6 |
I7 |
Ar-H -CH3 |
6.75 – 8.1 2.17 |
Multiplet Singlet |
CDCl3 |
Table No. 4
Anti-inflammatory activity
|
Sl No |
Name of the compounds |
Absorbance value (Mean ± SE) |
Inhibition of denaturation (in %) |
|
01 |
Control |
0.098 ± 0.001 |
-- |
|
02 |
Diclofenac Sod. |
0.209 ± 0.013 |
95.52 % |
|
03 |
P1 |
0.144 ± 0.012 |
50.00 % |
|
04 |
P2 |
0.141 ± 0.022 |
46.87 % |
|
05 |
P3 |
0.130 ± 0.013 |
35.42 % |
|
06 |
P4 |
0.163 ± 0.017 |
69.79 % |
|
07 |
P5 |
0.118 ± 0.023 |
22.99 % |
|
08 |
P6 |
0.148 ± 0.015 |
54.86 % |
|
09 |
P7 |
0.107 ± 0.016 |
11.45 % |
|
10 |
P8 |
0.127 ± 0.010 |
46.87 % |
|
11 |
I1 |
0.167 ± 0.018 |
63.10 % |
|
12 |
I2 |
0.098 ± 0.014 |
11.45 % |
|
13 |
I3 |
0.137 ± 0.023 |
35.42 % |
|
14 |
I4 |
0.179 ± 0.012 |
69.82 % |
|
15 |
I5 |
0.119 ± 0.016 |
23.95 % |
|
16 |
I6 |
0.097 ± 0.009 |
10.12 % |
|
17 |
I7 |
0.131 ± 0.013 |
36.46 % |
|
18 |
I8 |
0.142 ± 0.018 |
50.01 % |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









