About Authors:
Roshni Patel*, Prof. Arun Parikh, Prof. Vijayalakshmi Gudaparthi
Department of Pharmaceutical Chemistry, L.J.Institute of Pharmacy,
S.G.Highway, Ahmedabad-382210
*roshanimpharm@gmail.com
ABSTRACT
Medicinal chemistry involves chemical aspects of identification, and then systematic, thorough synthetic alteration of new chemical entities to make them suitable for therapeutic use. Indazoles have an important role in medicinal chemistry. Indazole nucleus is an important class of nitrogen containing heterocyclic widely used as key building block for the synthesis of various pharmaceutically important agents. Indazole possess wide range of biological activities such as bactericidal, fungicidal, analgesic, antihypertensive, anti-inflammatory and antitumor. In the present study we have synthesized some novel 3,3a,4,5-tetrahydro-2-(substituted benzenesulphonyl)-3-(substituted phenyl)-2H-benzo[g]indazoles, by condensation of 1-tetralone with different substituted aromatic aldehydes and the resulted 2-(substituted benzylidene)-3,4,-dihydronaphtalen-1-ones on reaction with hydrazine hydrate in methanol followed by treatment with different substituted sulfonyl chlorides in pyridine gave the titled compounds.The synthesized compounds were characterized by IR, NMR and Mass spectral analysis. All newly synthesized derivatives were evaluated for antibacterial activity against gram + ve and gram-ve bacteria B.cereus, S.aureus and E.coli respectivelyand antifungal activity againstC.albicans and all the synthesized compounds showed significant antibacterial and antifungal activity.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1385
INTRODUCTION
The indazole ring is an important pharmacophore in medicinal chemistry. Benzannulated nitrogen heterocycles are ubiquitous in pharmaceutical research. As part of a continuing effort to develop novel heterocyclic compounds with potential therapeutic biological activity, we are currently involved in the synthesis of a large number of indazole derivatives. Over the past decade, the indazole structural variant (benzo[c]pyrazole) has received much attention due to a broad range of biological activity. The synthesis of the indazole core, as well as the functionalization of the indazole ring system has recently been reviewed.1 Despite the many useful applications of indazole derivatives, indazole chemistry remains less studied compared to other heteroaromatic compounds, such as indole or benzimidazole. The indazole ring has two nitrogen atoms and presents annular tautomerism with regards to the position of the NH hydrogen atom. Due to the difference in energy between the tautomers, the 1H-tautomer (the benzenoid form) predominates in the gas-phase, solution and solid state, and its derivatives are usually thermodynamically more stable than the corresponding 2H-forms.
The regioselectivity of the reaction is also dependent on the nature of the alkylating agents used. The substitution at the different atoms of the six- and five membered rings with side chains with different length and functionalisation, can afford a large number of indazole derivatives, presenting a promising field to provide new derivatives with biological/therapeutical properties.2
Moreover, the increasing biological importance of indazole derivatives particularly in chemotherapy, promoted us to develop and synthesize new molecules with indazole moiety, with the aim of obtaining some novel heterocyclic systems with potentially enhanced biological properties. Indazoles constitute an important class of heterocycles that display interesting biological properties, such as anti-emetic 3,anticancer4, antimicrobial5, and anti-inflammatory activities6. The indazole ring system is also present in many other compounds such as herbicides, dyes or sweeteners like guanidine-1H indazole.
[adsense:468x15:2204050025]
Recently, indazole derivative7, ABT-102 (1), has been identified as a potent vanilloid receptor (VR1) antagonist. This compound is currently undergoing advanced clinical development for the treatment of chronic pain, Axitinib (2), was synthesized by Pfizer and WO/2007/056170 (3), was developed by Baeyer US as potent IGF-1R kinase inhibitors.
The synthesis of new hexahydroindazoles containing pharmacophore fragments and groups was carried out and their stereochemistry and bioactivity were investigated. The hexahydroindazole derivatives shared a larger part of the report for their use in pharmaceutical compositions as nonestrogenic antifertility agents and antihypertensive agents.
MATERIAL AND METHODS
Material:
The chemicals and reagentsused in the project work were of AR and LR grade, procured from Astron chemicals, Ahmedabad and they are used as they obtained.
Equipments:
Purity of compounds was checked by thin layer chromatography. Melting points of synthesized compounds were determined by open capillary method. The IR spectra of synthesized compounds were recorded on a Fourier-Transform IR spectrophotometer (model-DRS 8400, Shimadzu) in the range of 400-4000 cm-1 using KBr pellets. The 1H-NMR spectra of synthesized compounds were recorded on Bruker Avance II 500MHz FT-NMR spectrophotometer (TOPSPIN 1.3 version) using DMSO-d6 as solvent.Mass spectrum was recorded by MDS SCIEX API 2000 LCMS/MS (Applied Biosystems) instrument.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Method:
Step-1: Synthesis of (E)-2-(substituted benzylidene)-3,4-dihydronaphthalen-1-(2H)-one 8
The solution of substituted aromatic aldehyde (0.02 mole) and 1-tetralone (0.02 mole) in 18 ml PEG 600 was added to KOH (0.02 mole) and few ml of water. The reaction mixture was stirred for 1 hrs at room temperature. After stirring, the mixture was cooled in refrigerator overnight , filtered and washed with cooled ethanol, dried in air and recrystallized from ethanol.
Step-2: Synthesis of 3,3a,4,5-tetrahydro -3-(substituted phenyl) -2H- benzo [g] indazole
The solution of hydrazine hydrate (0.03 mole) and step-1 compound,(E)-2-(substituted benzylidene)-3,4-dihydronaphthalen-1-(2H)-one (0.01 mole) in methanol (100 ml) was refluxed for 3 hrs. The above reaction mixture was kept in refrigerator for 24 hrs. The precipitated product was filtered, washed with methanol and recrystallized from methanol.
Step-3: Synthesis of 3,3a,4,5-tetrahydro 2-(substituted benzene sulphonyl)-3-(substituted phenyl) -2H-benzo[g]indazole
To the solution of 3,3a,4,5-tetrahydro-3-(substituted phenyl)-2H-benzo[g]indazole (0.004 mole) in pyridine (20 ml) was added of substituted benzene sulphonyl chloride (0.004 mole), and the mixture was heated on a water bath for 3 hrs. Then the reaction mixture was cooled, poured into dilute hydrochloric acid and the solid thus obtained was filtered, washed with water and recrystallized from rectified spirit.
SCHEME
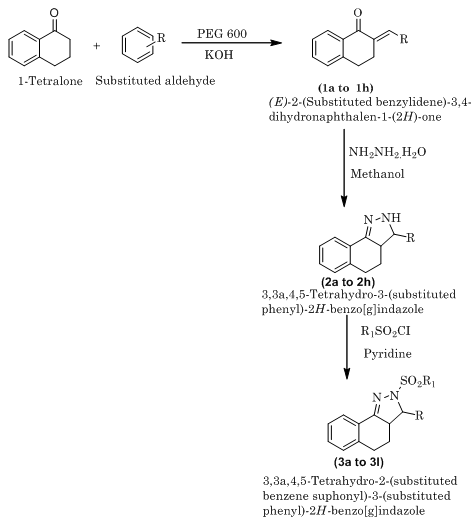
Table 1: Physical data table of synthesized compounds
|
No. |
Name of sSubstituent ((R) |
Name of substituent (R1) |
Molecular formula |
Melting point(°C) |
Yield % |
Rf Value |
|
1 |
4-Methoxy phenyl |
Benzene sulphonyl |
C24H19N2O3S |
188-1900C |
66% |
0.77 Toluene:Ethyl acetate (7:3) |
|
2 |
3,4-Dimethoxy phenyl |
Benzene sulphonyl |
C25H22N2O4S |
180-1820C |
71% |
0.48 Toluene:Ethyl acetate (5:5) |
|
3 |
Vanillin sulphonate
|
Benzene sulphonyl |
C30H24N2O6S2 |
118-1200C |
48% |
0.54 n-Hexane:Ethyl acetate (7:3) |
|
4 |
4-Chloro phenyl |
Benzene sulphonyl |
C22H17N5O2Cl |
186-1880C |
70% |
0.78 Toluene:Methanol (6:4) |
|
5 |
2-Nitro phenyl |
Benzene sulphonyl |
C22H17N3O4S |
148-1500C |
68% |
0.49 Toluene:Methanol (5:5) |
|
6 |
3-Niro phenyl |
Benzene sulphonyl |
C23H17N3O4S |
216-2180C |
55% |
0.65 Toluene:Ethyl acetate (7:3) |
|
7 |
2,3,4- Trimethoxy phenyl |
Benzene sulphonyl |
C23H17N3O4S |
170-1720C |
75% |
0.58 Toluene:Ethyl acetate (7:3) |
|
8 |
4-Dimethylamino phenyl |
Benzene sulphonyl |
C26H24N2O5S |
178-1800C |
56% |
0.62 Toluene:Ethyl acetate (5:5) |
|
9 |
Vanillin sulphonate |
2-Methoxy,5-carboxy benzene sulphonyl |
C32H28N2O9S2 |
110-1120C |
66% |
0.61 Toluene:Methanol (7:3) |
|
10 |
4-Methoxy phenyl |
2-Methoxy,5-carboxy benzene sulphonyl |
C26H24N2O6S |
86-890C |
52% |
0.55 Toluene:Ethyl acetate (6:4) |
|
11 |
3-Nitro phenyl |
2-Methoxy,5-carboxy benzene sulphonyl |
C25H21N3O7S |
102-1040C |
60% |
0.55 Toluene:Ethyl acetate (6:4) |
|
12 |
2,3,4- Trimethoxy phenyl |
2-Methoxy,5-carboxy benzene sulphonyl |
C28H28N2O8S |
68-700C |
72% |
0.55 Toluene:Ethyl acetate (5:5) |
(IIIa) 3,3a,4,5-Tetrahydro-2-(benzenesulphonyl)-3-(4-methoxyphenyl)-2H benzo[g] indazole
IR (KBr, cm-1):(3050) C-H str; (1425) C=C str; (1253) C-O-C str(sym); (1030)C-O-C str (asym) ; (1612) C=N str;.(1089) S=O str; (831) N-SO2 str ;1H NMR (δ ppm, DMSO): 3.8(S,3H,Ar-OCH3);1.5-2.6(M,5H,-CH2-CH2-CH);6.8-7.8(M,10H,Ar-H);4.1(S,1H,Ar-CH); Mass: (m/e) 418 (M+); 419.44; (M+1).
(IIIb)3,3a,4,5-Tetrahydro-2-(benzene sulphonyl)-3-(3,4-dimethoxyphenyl)-2H-benzo[g] indazole
IR (KBr, cm-1):(3045) C-H str; (1454) C=C str; (1263) C-O-C str(sym); (1022) C-O-C str (asym) ; (1595) C=N str; (1168) S=O str; (866) N-SO2 str;1H NMR (δ ppm, DMSO): 1.5-2.6 (M,5H,-CH2-CH2-CH);6.8-7.8(M-10H,Ar-H); 4.1(S,1H,Ar-CH) Mass: (m/e) 449.12;(M+1).
(IIIc) 5-(3,3a,4,5-Tetrahydro-2(benzene sulphonyl)-2H-benzo[g]indazole -3-yl)-2-methoxyphenyl benzenesulfonate
IR (KBr, cm-1):(3064) C-H str; (1508) C=C str; (1263) C-O-C str(sym); (1029)C-O-C str (asym) ; (1602) C=N str;.(1159) S=O str; (941) N-SO2 str ; 1H NMR (δ ppm, DMSO): 3.8( S,3H,Ar-OCH3);1.5-2.6(M,5H,-CH2-CH2-CH);6.8-7.8(M-15H,Ar-H);4.1(S,1H,Ar-CH)Mass: (m/e) 574 (M+),575-(M+1).
(IIId) 3,3a,4,5-Tetrahydro-2-(benzene sulphonyl)-3-(4-chlorophenyl)-2H-benzo[g] indazole
IR (KBr, cm-1):(3000) C-H str; (1492) C=C str; (798) C-Cl str ; (1039) C=N str;.(1091) S=O str; (823) N-SO2 str ; 1H NMR (δ ppm, DMSO): 1.5-2.6(M,5H,-CH2-CH2-CH);6.8-7.8(M,11-Ar-H);4.1(S,1H,Ar-CH); Mass: (m/e).424.11(M+2).
(IIIe) 3,3a,4,5-Tetrahydro-2-(benzene sulphonyl)-3-(2-nitrophenyl)-2H-benzo[g]indazole
IR (KBr, cm-1):(3058) C-H str; (1490) C=C str; (1253) ; (1602) C=N str; (1159) S=O str; (941) N-SO2 str ;
(IIIf)3,3a,4,5-Tetrahydro-2-(benzene sulphonyl)-3-(3-nitrophenyl)-2H-benzo[g]indazole
IR (KBr, cm-1): (3055) C-H str; (1480) C=C str; (1253) ; (1610) C=N str; (1162) S=O str; (955) N-SO2 str ; Mass: (m/e) 478 (M+2) 434.45-M+1
(IIIg) 3,3a,4,5-Tetrahydro-2-(benzene sulphonyl)-3-(2,3,4-trimethoxyphenyl)-2H-benzo [g]indazole
IR (KBr, cm-1):(3063) C-H str; (1482) C=C str; (1261) C-O-C str(sym); (1045) C-O-C str (asym) ; (1599) C=N str; (1174) S=O str; (829) N-SO2 str ;1H NMR (δ ppm, DMSO): 1.5-2.6(M,5H,-CH2-CH2-CH);6.8-7.8(M-12H,Ar-H);4.1(S,1H,Ar-CH); Mass: (m/e) 479.16 (M+1).
(IIIh) 4-(3,3a,4,5-Tetrahydro-)-2-(benzene sulphonyl)-2H-benzo[g]indazole-3-yl)-N,N-dimethyl benzenamine
IR (KBr, cm-1):(3055) C-H str; (1480) C=C str; (1610) C=N str; (1162) S=O str; (955) N-SO2 str ; Mass: (m/e) 431.45(M) ,432.17(M+1)
(IIIi)5-(3,3a,4,5-Tetrahydro-2-(2methoxy,5-carboxy benzene sulphonyl)-2H-benzo[g]indazole-3-yl)-2-methoxyphenyl benzenesulfonate
IR (KBr, cm-1):(3061) C-H str; (1504) C=C str; (1274) C-O-C str(sym); (1030)C-O-C str (asym) ; (1579) C=N str;.(1178) S=O str; (831) N-SO2 str ; (1637) C=O str.
(IIIj)3,3a,4,5-Tetrahydro-2-(2-methoxy,5-carboxy benzene sulphonyl)-3-(4-methoxyphenyl)-2H-benzo[g]indazole
IR (KBr, cm-1):(3053) C-H str; (1420) C=C str; (1240) C-O-C str(sym); (1034)C-O-C str (asym) ; (1628) C=N str;.(1089) S=O str; (828) N-SO2 str ;Mass: (m/e) 493.51(M+1).
(IIIk)3,3a,4,5-Tetrahydro-2-(2-methoxy,5-carboxy benzene sulphonyl)-3-(3-nitrophenyl) -2H-benzo[g]indazole
1H NMR (δ ppm, DMSO): 3.8( S,3H,Ar-OCH3);1.5-2.6(M,5H,-CH2-CH2-CH);6.8 7.8(M,12H,Ar-H);4.1(S,1H,Ar-CH); Mass: (m/e) 508.1(M+1)
(IIIl)3,3a,4,5-Tetrahydro-2-(2-methoxy,5-carboxy benzene sulphonyl)-3-(2,3,4 trimethoxyphenyl)-2H benzo[g]indazole
IR (KBr, cm-1):(3063) C-H str; (1496) C=C str; (1240) C-O-C str(sym); (1045)C-O-C str (asym) ; (1599) C=N str; Mass: (m/e) 552.18-(M),553.18(M+1)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSION
Biological Evaluations Microbial Screening:
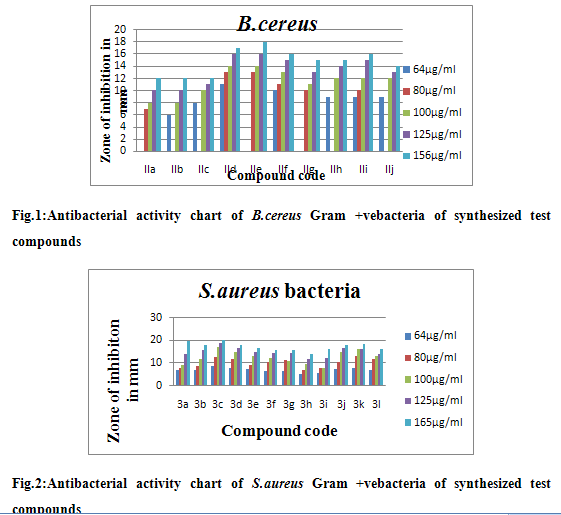
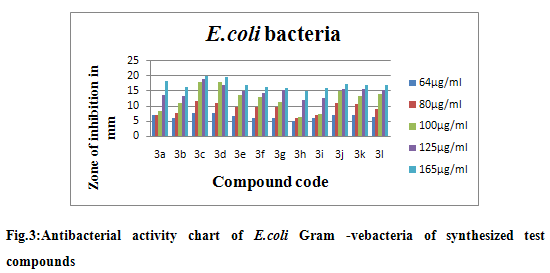
The synthesized compounds were screened for their anti-microbial activity by Agar diffusion method and Disc diffusion method using medium F and Sabouraud’s agar medium for bacteria and fungi respectively. Antimicrobial activity was evaluated by measuring the diameter of zone of inhibition against test organisms. Based on the results it is refered that synthesis of some indazole derivatives have significant inhibition effect on the growth of bacteria like Bacillus cereus, Staphylococus aureus and Escherichia coli and fungi likeCandida albicans. The results were tabulated in table. The results showed that the compound IIIc, IIId, and IIIl showed significant antibacterial activity when compare to that the standard (Streptomycin and Tetracycline) and compound IIIc and IIId showed significant antifungal activity when compare to that the standard (Miconazole).
Table-2 Antibacterial activity (zones of inhibition) of synthesised compound
|
Compound code |
Conc(µg/mL) |
Zone of inhibition(mm) |
|||
|
Gram +ve |
Gram -ve |
||||
|
B.Cereus |
S.aureus |
E.coli |
|||
|
3a |
64 |
7.10 |
7.0 |
6.93 |
|
|
80 |
8.4 |
8.1 |
7.12 |
||
|
100 |
9.45 |
9.3 |
8.18 |
||
|
125 |
14.32 |
13.98 |
13.52 |
||
|
165 |
19.45 |
19.58 |
18.40 |
||
|
3b |
64 |
7.2 |
6.9. |
6.1 |
|
|
80 |
9.66 |
8.7 |
7.71 |
||
|
100 |
12.75 |
11.9 |
10.87 |
||
|
125 |
15.44 |
15.7 |
13.2 |
||
|
165 |
19.55 |
18.2 |
16.4 |
||
|
3c |
64 |
9.2 |
8.9 |
7.8 |
|
|
80 |
12.86 |
12.7 |
11.68 |
||
|
100 |
17.20 |
17.1 |
17.98 |
||
|
125 |
19.6 |
18.9 |
18.92 |
||
|
165 |
22.1 |
20.1 |
20.1 |
||
|
3d |
64 |
9 |
7.9 |
7.5 |
|
|
80 |
12.5 |
12 |
10.9 |
||
|
100 |
16.7 |
14.9 |
16.9 |
||
|
125 |
17.5 |
16.8 |
18.1 |
||
|
165 |
21.5 |
18.1 |
19.7 |
||
|
3e |
64 |
8.2 |
7.6 |
6.5 |
|
|
80 |
11.2 |
9.2 |
9.8 |
||
|
100 |
14.2 |
13.4 |
13.8 |
||
|
125 |
16.24 |
14.9 |
15 |
||
|
165 |
18.1 |
16.8 |
16.9 |
||
|
3f |
64 |
7.8 |
6.8 |
5.9 |
|
|
80 |
10.9 |
10.8 |
9.8 |
||
|
100 |
13.6 |
12.2 |
13.1 |
||
|
125 |
15.9 |
14.4 |
14.2 |
||
|
165 |
17.85 |
16 |
16.4 |
||
|
3g |
64 |
7.5 |
6.5 |
6.12 |
|
|
80 |
11.66 |
11.5 |
9.8 |
||
|
100 |
12.1 |
10.9 |
11.3 |
||
|
125 |
15.23 |
14.5 |
15.5 |
||
|
165 |
16.9 |
15.7 |
15.9 |
||
|
3h |
64 |
6.55 |
5.5 |
4.9 |
|
|
80 |
7.81 |
6.9 |
5.9 |
||
|
100 |
8.26 |
9.8 |
6.33 |
||
|
125 |
13.4 |
12 |
12.1 |
||
|
165 |
18.5 |
14.3 |
15 |
||
|
3i |
64 |
6.85 |
5.9 |
5.9 |
|
|
80 |
8.0 |
7.8 |
6.89 |
||
|
100 |
8.25 |
8.1 |
7.2 |
||
|
125 |
13.8 |
12.4 |
12.5 |
||
|
165 |
18 |
16.4 |
15.88 |
||
|
3j |
64 |
8.4 |
7.4 |
7.1 |
|
|
80 |
11.6 |
10.5 |
10.9 |
||
|
100 |
15.82 |
14.8 |
14.9 |
||
|
125 |
17.2 |
16.9 |
15.7 |
||
|
165 |
19.6 |
18 |
17.5 |
||
|
3k |
64 |
8.7 |
7.8 |
6.88 |
|
|
80 |
12 |
13.4 |
10.75 |
||
|
100 |
16.22 |
17 |
13.2 |
||
|
125 |
17.2 |
16.3 |
15.8 |
||
|
165 |
19 |
18.7 |
16.9 |
||
|
3l |
64 |
7.11 |
7 |
6.3 |
|
|
80 |
11.04 |
12.1 |
9 |
||
|
100 |
12.5 |
13.2 |
14 |
||
|
125 |
15.2 |
14 |
15.2 |
||
|
165 |
19.5 |
16.4 |
17.1 |
||
|
Streptomycin |
25 |
15 |
12.9 |
- |
|
|
50 |
19.7 |
18 |
- |
||
|
75 |
21.8 |
19.5 |
- |
||
|
100 |
24.3 |
21 |
- |
||
|
Tetracycline |
25 |
- |
- |
13.5 |
|
|
50 |
- |
- |
15.2 |
||
|
75 |
- |
- |
18.7 |
||
|
100 |
- |
- |
22 |
||
Degree of activity was measured by the zone of inhibition (mm), (--) No inhibition
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table-3 Antifungal activity (zones of inhibition) of synthesised compounds
|
Compound code |
Conc. (µg/ml) |
Zone of inhibition (mm) |
Compound code |
Conc. (µg/ml) |
Zone of inhibition (mm) |
|
C. albicans |
C. albicans |
||||
|
3a |
50 |
4.5 |
3g |
50 |
6.1 |
|
100 |
7.9 |
100 |
9.7 |
||
|
150 |
8.3 |
150 |
10.4 |
||
|
200 |
14.1 |
200 |
15.7 |
||
|
3b |
50 |
5.6 |
3h |
50 |
2.9 |
|
100 |
10.7 |
100 |
5.9 |
||
|
150 |
16.3 |
150 |
10.7 |
||
|
200 |
14.6 |
200 |
15.1 |
||
|
3c |
50 |
6.3 |
3i |
50 |
4.7 |
|
100 |
13.5 |
100 |
11.4 |
||
|
150 |
16.0 |
150 |
13.5 |
||
|
200 |
18.2 |
200 |
13.7 |
||
|
3d |
50 |
6.9 |
3j |
50 |
5.5 |
|
100 |
8.5 |
100 |
9.2 |
||
|
150 |
10.7 |
150 |
10.6 |
||
|
200 |
18.5 |
200 |
13.5 |
||
|
3e |
50 |
6.3 |
3k |
50 |
8.7 |
|
100 |
9.1 |
100 |
9.9 |
||
|
150 |
10.9 |
150 |
11.8 |
||
|
200 |
18 |
200 |
16.7 |
||
|
3f |
50 |
8.4 |
3l |
50 |
9.9 |
|
100 |
12.5 |
100 |
11.2 |
||
|
150 |
14.0 |
150 |
13.8 |
||
|
200 |
16.4 |
200 |
15.9 |
||
|
Miconazole |
25 |
9.7 |
Miconazole |
75 |
15.3 |
|
50 |
12.1 |
100 |
20.4 |
Degree of activity was measured by the zone of inhibition (mm), (--) No inhibition
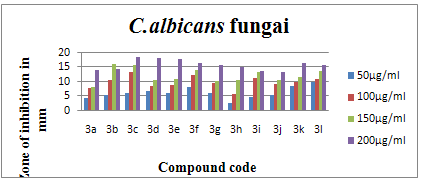
Fig.5:Antifungal activity chart of C. albicans fungiof synthesized test compounds.
CONCLUSION
Among all synthesized compounds, most of them showed significant antibacterial activity within the series against both of Gram +ve and Gram –ve bacteria at 125 and 156 μg/ml concentrations and antifungal activity at 150 and 200 μg/ml concentrations.
ACKNOWLEDGEMENTS
The authors are thankful to L.J.Institute of Pharmacy for providing facilities for synthesis and biological screening.
REFERENCES
1. Salovich J M, Lindsley C W, Hopkins C R: Synthesis of 1,3-diarylsubstituted indazoles utilizing a Suzuki cross-coupling /deprotection/ N-arylation sequence.Tetrahedron.Lett.2010;51: 3796–3799
2. Teixeira F C, Ramos H, Antunes I F,M. Curto M J ,Duarte M T ,Bento I: Synthesis and structural characterization of 1- and 2-substituted indazoles: ester and carboxylic acid derivatives.Molecules,2006; 11:867-889
3. Rodgers J D, Johnson B L, Wang H, Greenberg R A, Erickson-Viitanen S, Klabe R M, Cordova B C, Rayner M M, Lam G N, Chang C H: Potent cyclic urea HIV protease inhibitors with benzofusedheterocycles as P2/P2′ groups.Bioorg. Med. Chem. Lett. 1996, 6,2919-2924; (b) Sun J H, Teleha C A, Yan J S, Rodgers J D, Nugiel D A: Efficient synthesis of 5-(bromomethyl)- and 5-(aminomethyl)-1H-indazole. J. Org. Chem.1997; 62: 5627-5629
4. Yates C M, Brown PJ, Stewart E L, Patten C, Austin J H, Holt J A, Maglich J M, Angell D C, Sasse RZ, Taylor S J, Uings I J, Trump R P: Structure guided design of 5-arylindazole glucocorticoid receptor agonists and antagonists.J. Med. Chem. 2010;53: 4531-4544
5. Dessole G, Branca D, Ferrigno F, Kinzel O, Muraglia E, Palumbi M. C,Rowley M, Serafini S, Steinkuhler C, Jones P: Discovery of N-[(1-aryl-1H-indazol-5-yl)methyl] amides derivatives as smoothned antagonists for inhibition of the hedgehog pathway.Bioorg.and Med. Chem.Lett. 2009;19: 4191-4195.
6. Selwood D L, Brummell D G, Budworth J, Burtin G E, Campbell R O, Chana S S, Charles I G, Fernandez P A, Glen R C, Goggin M C, Hobbs A J, Kling MR, Liu Q, Madge D. J, Meillerais S, Powell K. L,Reynolds K, Spacey G. D, Stables J N, Tatlock M A, Wheeler K. A, Wishart G, Woo C K: Synthesis and biological evaluation of Novel pyrazoles and Indazoles as activators of Nitric oxide receptor,soluble GuanylateCyclase. J. Med. Chem. 2001; 44:78-93.
7. Lukin K, Hsu M C, Fernando D, Leanna M R: New practical synthesis of indazoles via condensation of o-flurobenzaldehyde and their o-methyloximes with hydrazine.J. Org. Chem.,2006;71:8166-8172
8. Kamakshi R,Latha S S,Reddy BSR: An efficient synthesis of bio-active flurescent benzylidene tetralones.Indian J. Chem.2010;49B:944-947
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









