{ DOWNLOAD AS PDF }
 ABOUT AUTHOR
ABOUT AUTHOR
Dhrubo Jyoti Sen
Department of Pharmaceutical Chemistry,
Shri Sarvajanik Pharmacy College,
Mehsana, Gujarat, India
dhrubosen69@yahoo.com
ABSTRACT: Our goal is to perform in-vitro biotransformation of Prodrugs of ibuprofen+paracetamol, diclofenac sodium+paracetamol and ibuprofen+diclofenac sodium by acidic and alkaline hydrolysis of both ester (–COO–) and amide (–CONH–) linkages into free drugs and chromatographically separation of their Rt in HPLC. Since both ester (–COO–) and amide (–CONH–) linkages are susceptible for hydrolysis in both acidic pH (gastric pH) and basic pH (intestinal pH) to produce parent drug ibuprofen, diclofenac and paracetamol by biotransformation in in-vivo; so it will be implemented as a Prodrug which can show prolong action on pain and fever after getting release into free parent drug by biotransformation. The HPLC (High Performance Liquid Chromatography) study reports the retention time (Rt) and release kinetics of three Prodrugs by taking HPLC degradation datas of three samples of Prodrugs and individual HPLC datas of parent drugs separately to compare the Rt value of release of three drugs from Prodrugs in both acidic and alkaline pH. Prodrug–A (logP=4.56) releases Ibuprofen & Paracetamol, Prodrug–B (logP=4.90) releases Diclofenac & Paracetamol and Prodrug–C (logP=6.13) releases Ibuprofen & Diclofenac. This is a comparison study of drug release in in-vitro gastric as well as intestinal pH focusing on in-vivo biotransformation. Keywords: Prodrug-A/Prodrug-B/Prodrug-C, Ibuprofen, Paracetamol, Diclofenac sodium, Molecular weight, logP, UV λmax, IR, Mobile phase, TLC-Rf value, HPLC-Rt value, LOD, LOC
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2522
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 8 Received On: 23/05/2017; Accepted On: 23/05/2017; Published On: 01/08/2017 How to cite this article: Sen DJ;Study of in-vitro biotransformation of prodrugs of Ester and Amide linkages of Ibuprofen, Diclofenac Sodium and Paracetamol in acidic and alkaline medium; PharmaTutor; 2017; 5(8); 49-65 |
INTRODUCTION: Three Prodrugs (Prodrug–A, Prodrug–B and Prodrug–C) have been successfully synthesized and have shown different melting points from individual parent drugs (Ibuprofen, Diclofenac sodium and Paracetamol) which indicates the authenticity of fulfillment of Prodrug synthesis. [Jalpa G. Patel and Prof. Dr. Dhrubo Jyoti Sen; Synthesis of Prodrug of ester and amide linkages of NSAID having carboxylic acid, phenolic and imino groups: World Journal of Pharmacy and Pharmaceutical Sciences: 5(11), 897-908, 2016. (ISSN: 2278-4357, Impact Factor: 6.647, Index Copernicus Value: 52.51); Dhrubo Jyoti Sen and Jalpa G. Patel; Logarithmic partition coefficient comparison study and molecular weight of synthesized Prodrugs of ibuprofen+paracetamol, diclofenac sodium+paracetamol and ibuprofen+diclofenac sodium: American Journal of Advanced Drug Delivery: 4(05), 064-068, 2016. (ISSN: 2321-547X, Impact Factor: 0.786)].[1,2]
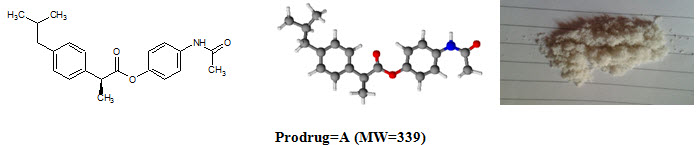
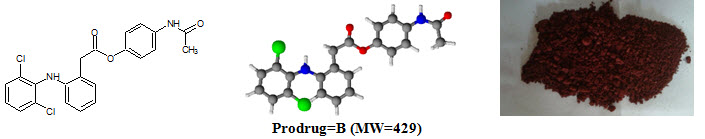
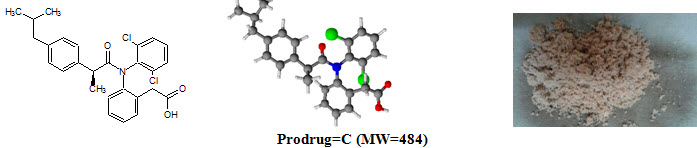
Figure-1: Prodrugs
The synthesized Prodrugs were characterized by m.p., logP values and IR (Infra Red) spectrum for structural identification. Their solubility parameters also found different from parent drugs. Prodrug is a substance having no medicinal importance but after biotransformation in GIT it releases the parent drug which is able to show the pharmacological activity.[3-5]
Ibuprofen [C13H18O2; MW=206] (2–[4–(2–methylpropyl)phenyl]propanoic acid), from isobutylphenylpropanoic acid, is a drug in the nonsteroidal anti–inflammatory drug (NSAID) class used for treating pain, fever and inflammation. logP=3.97, pKa=4.91. Ibuprofen m.p.=theoretical (76°C), practical (75°C).
Diclofenac sodium [NaC14H10Cl2NO; MW=302] (Sodium {2–[(2,6–dichlorophenyl)amino]phenyl}acetic acetate) is a nonsteroidal anti–inflammatory drug (NSAID) taken or applied to reduce inflammation and as an analgesic reducing pain in certain conditions. logP=4.51, pKa=4.15. Diclofenac sodium m.p.=theoretical (283–285°C), practical (282°C)
Paracetamol [C8H9NO2; MW=151] (N–(4–hydroxyphenyl)acetamide), also known as acetaminophen or APAP, is a medication used to treat pain and fever. logP=0.46, pKa=9.38. Paracetamol m.p.=theoretical (169°C), practical (170°C).
Experimental part:
Mobile phase of TLC values has been selected after trial and error for all individual NSAID (ibuprofen, diclofenac sodium and paracetamol) according to their logP and Rf values have been determined and after that Rf values have been obtained for all three Prodrugs (Prodrug-A, Prodrug-B, Prodrug-C). Mobile phase solvent ratio of TLC helped to select the mobile phase selection for HPLC.[6-8] The values are as follows:

UV maximum Absorbance (Prodrug-A)

UV maximum Absorbance (Prodrug-B)
UV maximum Absorbance (Prodrug-C)
Figure-2: λmax of Prodrugs
|
Samples |
Molecular Formula |
% yield |
Melting Point (°C) |
Molecular Weight |
λmax (nm) |
logP |
|
Prodrug-A |
C21H25NO3 |
83.67 |
152–155°C |
339 |
275 |
4.56 |
|
Prodrug-B |
C22H18Cl2N2O3 |
88.65 |
220–222°C |
429 |
262 |
4.90 |
|
Prodrug-C |
C27H27Cl2NO3 |
91.32 |
100–105°C |
484 |
276 |
6.13 |
Table-1: Physicochemical parameters of Prodrugs
Solubility study of Prodrug-A, Prodrug-B and Prodrug-C:
The solubility of Prodrug-A, Prodrug-B and Prodrug-C were practically determined. Solubility was determined by taking 100mg of Prodrug-A, Prodrug-B and Prodrug-C respectively in 10ml volumetric flask, adding required quantity of solvent at room temperature and shaken for few minutes.[9-11] Solubility data for each study was observed and recorded in Table-2.
|
Solvent |
Solubility |
|
Water |
Prodrug-A: Soluble, Prodrug-B: Insoluble, Prodrug-C: Slightly soluble |
|
Methanol |
Freely soluble |
|
Acetone |
Freely soluble |
Table-2: Solubility parameters of Prodrugs
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Instrument:
A double beam UV visible spectrophotometer: Manufacturer: Shimadzu. Model: UV-1800, Shimadzu, Japan.
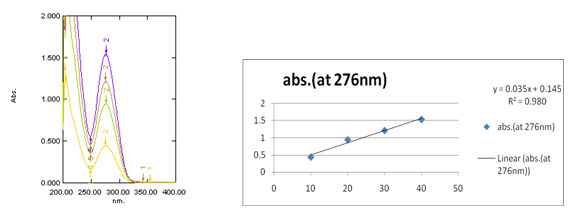
Preparation of Standard solution of Prodrug-A: Weighed accurately about 100mg of Prodrug-A separately transferred quantitatively to 100ml volumetric flask. Dissolved in about 70ml of Methanol by sonication and diluted to volume with methanol and mixed. Transferred 0.1ml of this solution to 10ml volumetric flask, diluted to volume with diluents (methanol) and mixed (10µg/ml) and absorption was observed at 275nm.[12] The values of three Prodrugs were given in the Table-3.
|
Prodrug-A |
Conc. (μg/ml) |
Abs. |
Prodrug-B |
Conc. (μg/ml) |
Abs. |
Prodrug-C |
Conc. (μg/ml) |
Abs. |
|
10 |
0.112 |
10 |
0.208 |
10 |
0.441 |
|||
|
20 |
0.222 |
20 |
0.438 |
20 |
0.945 |
|||
|
30 |
0.338 |
30 |
0.664 |
30 |
1.216 |
|||
|
40 |
0.483 |
40 |
0.845 |
40 |
1.536 |
Table-3: Absorption datas of Prodrug-A, Prodrug-B and Prodrug-C
Infra Red Spectral studies of Prodrug-A, Prodrug-B and Prodrug-C:
IR spectras of three Prodrugs were measured in KBr pellets in Shimadzu FT-IR spectrophotometer and values in cm-1 were obtained to interpretation of structural framework.
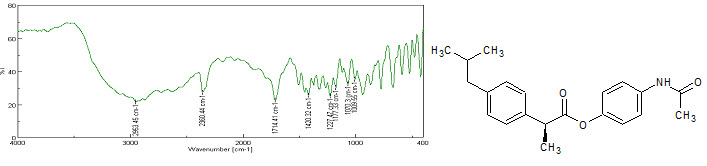
Figure-3: IR Spectra of Prodrug-A
Prodrug-A: IR (cm–1; ν):
CH3 asym. stretch: (standard=2970-2950/2880-2860, found=2953.45), >C=O stretching: (standard=1725-1705, found=1714.41), Amide II band: (standard=1680-1630, found=1509.03), CH-CO deformation: (standard=1470-1430/1380-1370, found=1420.32), C=C stretching: (standard=1615–1580, found=1610), C-N-H group: (standard=1090–1020, found=1070.3), Para-disubstituted aromatic ring: (standard=860–800, found=850).[13]

Figure-4: IR Spectra of Prodrug-B
Prodrug-B: IR (cm–1; ν):
N-H stretching: (standard=3500-3100, found=3343), C-H stretching (aromatic): (standard=3150-3050, found=3136.36), C=C stretching: (standard=1615–1580, found=1621), C-N stretching: (standard=1650–1550, found=1580), >C=O stretching: (standard=1725-1705, found=1721), C-CO-C stretching: (standard=1320-1210, found=1284.63).[14]
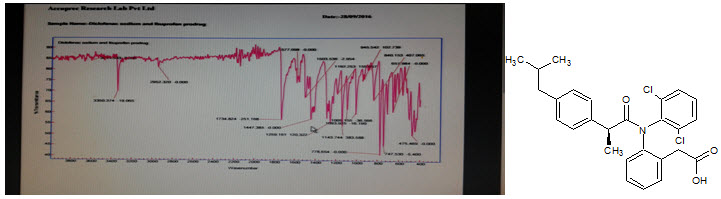
Figure-5: IR Spectra of Prodrug-C
Prodrug-C: IR (cm–1; ν): Amide (–CONH–): (standard=1630–1680, found=1735), Ketone (>C=O): (standard=1705–1725, found=1735), Imino (–NH–): (standard: 3350–3320, found=3350), Chlorine (–Cl): (standard=700-800, found=747, 778), Phenyl (–C6H5): (standard=1615–1580, found=1577), Carboxylic acid (–COOH): (standard=1700–1725, found=1734), Methyl (CH3–): (standard=2970–2950/2880–2860, found=2952) Methyl C–H asym./sym. stretch standard=1470–1430/1380–1370, found=1447).[15]
Development and validation of Stability Indicating RP-HPLC Method for Prodrug-A (Ibuprofen and Paracetamol)
Experimental: Reagents and Materials, Prodrug-A synthesized in our college lab, Methanol (HPLC grade, Finar Chemicals Ltd, Ahmedabad, India), Water (HPLC grade, Finar Chemicals Ltd, Ahmedabad, India).
Equipments and Instruments: Shimadzu HPLC instrument (LC-2010 CHT) equipped with prominence diode array detector (SPD-M20A) (Software LC Solution), Analytical balance (Acculab ALC-2014, Huntingdon Valley, PA), Ultra sonicator (EN 30 US, Enertech Fast Clean, Mumbai, India), Hot air oven (TO-90S, Thermolab, Mumbai, India), pH meter (Thermo Electron Corp., Pune, India)
Development and Optimization of RP-HPLC Method:
a) Selection of Wavelength: The sensitivity of HPLC method that used UV detection depends upon proper selection of detection wavelength. An ideal wavelength is the one that gives good response for the drugs that are to be detected. The λmax of Prodrug-A was 275nm in methanol, Prodrug-B was 262nm in methanol and Prodrug-C was 276nm in methanol.[16]
b) Selection of Chromatographic Conditions: Proper selection of the HPLC method depends upon the nature of the sample (ionic or ionisable or neutral molecule), its molecular weight, pKa and solubility. RP-HPLC was selected for the initial separation based on literature survey and its simplicity and suitability. To optimize the chromatographic conditions the effects of chromatographic variables such as mobile phase, pH, flow rate and solvent ratio were studied and the chromatographic parameters such as capacity factor, asymmetric factor, resolution and column efficiency were calculated. The pH of gastric acid varies from 1.5-3.5 in the human stomach lumen, the acidity being maintained by the proton pump H+/K+ ATPase. So the pattern for acid hydrolysis was adjusted at pH=3-3.5 by HCl. The pH of intestine varies from 5.6-6.9, so the pattern for alkaline hydrolysis was adjusted at pH=7.0-8.0 by NaOH. Finally the condition was chosen that gave the best resolution, symmetry and capacity factor was selected for estimation of Prodrug-A, Prodrug-B and Prodrug-C.[17]
c) Selection of Ratio of Mobile phase: The solution containing 100µg/ml of Prodrug-A, Prodrug-B and Prodrug-C respetively was chromatographed with mobile phase of different ratio of methanol and water.[18]
|
Prodrug-A |
Trials |
Ratio |
Remark |
Prodrug-B |
Trials |
Ratio |
Remark |
Prodrug-C |
Trials |
Ratio |
Remark |
|
1 |
Methanol: Water (60:40) |
Tailing |
1 |
ACN: Water (80:20) |
Tailing |
1 |
Methanol: Water (90:10) |
Tailing |
|||
|
2 |
Methanol: Water (70:30) |
Tailing |
2 |
ACN: Water (70:30) |
Tailing |
2 |
Methanol: Water (85:15) |
Tailing |
|||
|
3 |
ACN: Water (60:40) |
Tailing |
3 |
ACN: Methanol (80:20) |
Tailing |
3 |
Methanol: Water (70:30) |
Tailing |
|||
|
4 |
ACN: Water (70:30) |
Tailing |
4 |
ACN: Methanol (70:30) |
Tailing |
4 |
Methanol: Water (80:20) |
Symmetrical peak |
|||
|
5 |
Methanol: Water (80:20) |
Symmetrical peak |
5 |
Methanol: Water (80:20) |
Tailing |
|
|||||
|
|
6 |
Methanol: Water (70:30) |
Symmetrical peak |
||||||||
Table-4: Selection of mobile phase for Prodrug-A, Prodrug-B and Prodrug-C
Procedure for Solution preparation for Prodrug-A:
1A. Preparation of synthesized drug stock solution: Accurately weighed 100mg (0.1gm) of Prodrug-A was transferred in to 100ml volumetric flask, dissolved and diluted up to mark with diluents to yield a stock solution having concentration of 1000µg/ml Prodrug-A.[19]
2A. Preparation of working solution containing Prodrug-A: From the above solution (1000µg/ml of Prodrug-A) 0.2, 0.4, 0.6, 0.8, 1.0, 1.2ml was taken into 10ml volumetric flask to get concentration 20, 40, 60, 80, 100, 120µg/ml of Prodrug-A respectively.[20]
Procedure for Solution preparation for Prodrug-B:
1B. Preparation of synthesized drug stock solution: Accurately weighed 100mg (0.1gm) of Prodrug-B was transferred in to 100ml volumetric flask, dissolved and diluted up to mark with diluents to yield a stock solution having concentration of 1000µg/ml Prodrug-B.[21]
2B. Preparation of working solution containing Prodrug-B: From the above solution (1000µg/ml of Prodrug-B), 0.01, 0.05, 0.10, 0.15, 0.20, 0.25ml was taken in to10ml volumetric flask to get concentration 1, 5, 10, 15, 20, 25µg/ml of Prodrug-B respectively.[22]
Procedure for Solution preparation for Prodrug-C:
1C. Preparation of synthesized drug stock solution: Accurately weighed 100mg (0.1gm) of Prodrug-C was transferred in to 100ml volumetric flask, dissolved and diluted up to mark with diluents to yield a stock solution having concentration of 1000µg/ml Prodrug-C.[23]
2C. Preparation of working solution containing Prodrug-C: From the above solution (1000µg/ml of Prodrug-C), 0.1, 0.15, 0.2, 0.25, 0.3, 0.35ml was taken in to 10ml volumetric flask to get concentration 10, 15, 20, 25, 30, 35µg/ml of Prodrug-C respectively.[24]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Method Validation for Prodrug-A:
1A. System suitability test parameters: System suitability tests are used to verify that the resolution and repeatability of the system were adequate for the analysis intended. The parameters used in this test were the chromatographic peak resolution, theoretical plate number and tailing factor. The repeatability of these parameters was checked by solutions of Prodrug-A.[25]
2A. Linearity and Range: Aliquots of stock solution (0.2, 0.4, 0.6, 0.8, 1.0, 1.2ml) were transferred into series of 10ml volumetric flasks and diluted up to mark with mobile phase. This yielded solution of 20, 40, 60, 80, 100, 120µg/ml of Prodrug-A. An aliquot of 10µl of each solution was injected under operating chromatographic condition. Calibration curve of area versus respective concentration was plotted and correlation co-efficient and regression line equation for Prodrug-A was calculated. Each response was an average of three determinations.[26]
Method Validation for Prodrug-B:
1B. System suitability test parameters: System suitability tests are used to verify that the resolution and repeatability of the system were adequate for the analysis intended. The parameters used in this test were the chromatographic peak resolution, theoretical plate number and tailing factor. The repeatability of these parameters was checked by solutions of Prodrug-B.[27]
2B. Linearity and Range: Aliquots of stock solution (0.01, 0.05, 0.10, 0.15, 0.20, 0.25ml) were transferred into series of 10ml volumetric flasks and diluted up to mark with mobile phase. This yielded solution of 1, 5, 10, 15, 20, 25µg/ml of Prodrug-B. An aliquot of 10µl of each solution was injected under operating chromatographic condition. Calibration curve of area versus respective concentration was plotted and correlation co-efficient and regression line equation for Prodrug-B was calculated. Each response was an average of three determinations.[28]
Method Validation for Prodrug-C:
1C. System suitability test parameters: System suitability tests are used to verify that the resolution and repeatability of the system were adequate for the analysis intended. The parameters used in this test were the chromatographic peak resolution, theoretical plate number and tailing factor. The repeatability of these parameters was checked by solutions of Prodrug-C.[29]
2C. Linearity and Range: Aliquots of stock solution (0.1, 0.15, 0.2, 0.25, 0.3, 0.35ml) were transferred into series of 10ml volumetric flasks and diluted up to mark with mobile phase. This yielded solution of 10, 15, 20, 25, 30, 35µg/ml of Prodrug-C. An aliquot of 10µl of each solution was injected under operating chromatographic condition. Calibration curve of area versus respective concentration was plotted and correlation co-efficient and regression line equation for Prodrug-C was calculated. Each response was an average of three determinations.[30]
Standard Paracetamol peak at 230nm in mobile phase Phosphate Buffer:ACN (40:60v/v):-
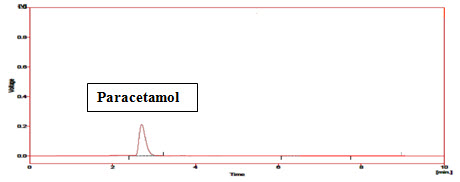
Figure-6: Chromatogram of Standard Paracetamol (Rt=2.3min)
Paracetamol releases first (Rt=2.3min) because it’s logP is 0.46 (highly polar).
Standard Ibuprofen peak at 278 nm in mobile phase Water:ACN (80:20v/v):
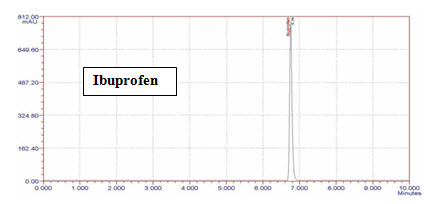
Figure-7: Chromatogram of Standard Ibuprofen (Rt=6.8min)
Ibuprofen releases slow (Rt=6.8min) because it’s logP is 3.97 (nonpolar).
Standard Diclofenac Sodium peak at 284nm in mobile phase ACN:triethylamine buffer (50:50v/v):-
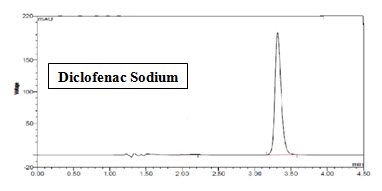
Figure-8: Chromatogram of Standard Diclofenac Sodium (Rt=3.4min)
Diclofenac sodium releases moderate (Rt=3.4min) because it’s logP is 4.51 (semipolar).
Optimized Chromatographic Conditions for Prodrug-A: HPLC system: LC 2010 CHT (Shimadzu), PDA detector (PDA-SPD-M10AVP, Shimadzu), Column (Stationary Phase): Kromasil C18 (150mm×4.6mm, 5µm particle size), Mobile phase : (Methanol: Water)(80:20 v/v), Flow Rate: 1.0 ml/min, Detection Wavelength: 275nm, Column oven Temp: 40ºC, Run time: 10 mins. Diluent: All the final dilution of sample was done with methanol.[31]
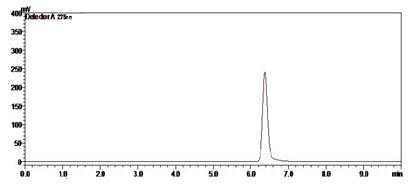
Figure-9: Chromatogram of Prodrug A (100µg/ml) (Rt=6.38min)
Prodrug-A has logP 4.56 so release rate is higher.
Value of System suitability Parameter: Retention time (min.): 6.38, Theoretical plates: 17967.194, Tailing factor: 1.396, Resolution: 5.567, Capacity factor: 3.3.
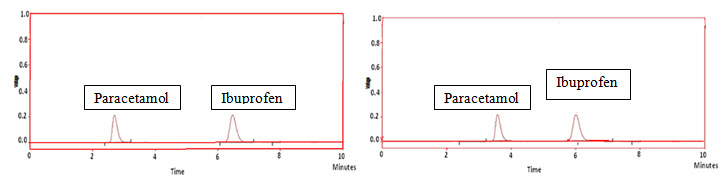
Figure-10: Chromatogram of Prodrug-A in Acidic medium (HCl: pH=3.5) and in Alkaline medium (NaOH: pH=8.0)
Paracetamol has Rt=2.5min in acidic medium and 3.5min in alkaline medium; Ibuprofen has Rt=6.35min in acidic medium and 6.15min in alkaline medium.
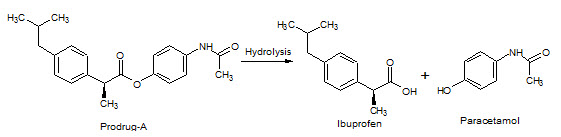
Figure-11: Prodrug–A hydrolysis into Ibuprofen & Paracetamol
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Method Validation:
Linearity and Range: Overlain chromatogram of Prodrug-A was shown in Figure-12. The linearity of Prodrug-A was found to be in the range of 20-120µg/ml with correlation coefficient 0.999 as shown in Figure-13.[32]
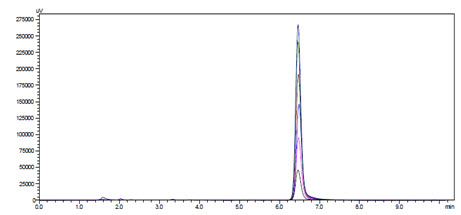
Figure-12: Overlain Linearity Chromatogram of Prodrug-A (20-120µg/ml)
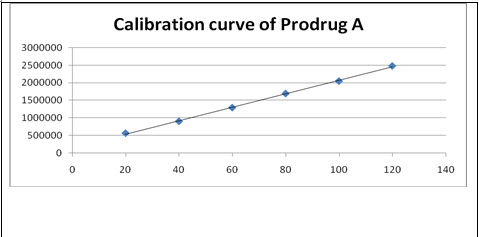
Figure-13: Calibration Curve of Prodrug-A
Precision:
1.Repeatability (Intra-day precision): The data for Intra-day precision for Prodrug-A is shown in Table-6. The %RSD for Intra-day precision was found to be 0.35-1.50 % for Prodrug-A.
2. Inter-day Precision (different days): The data for Inter-day Precision for Prodrug-A is shown in Table-6. The %RSD Inter-day Precision for was found to be 0.17-0.29 % for Prodrug-A.[33]
Intra-day precision data and Inter-day precision data for estimation of Prodrug-A:
|
Intra-day precision |
Conc.(µg/ml) |
Mean peak Area±S.D. |
%RSD |
Inter-day precision |
Conc.(µg/ml) |
Mean peak Area±S.D. |
%RSD |
|
60 |
1287673±6094.77 |
0.47 |
60 |
931933±2776.952 |
0.29 |
||
|
80 |
1690409±6061.18 |
0.35 |
80 |
1891173±3295.832 |
0.17 |
||
|
100 |
2033542±68803.05 |
1.50 |
100 |
2694467±6749.519 |
0.25 |
*= Average of three determinations
Table-6: Comparison of Intra-day precision data and Inter-day precision data for estimation of Prodrug-A
LOD and LOQ: The Limit of Detection (LOD) was found to be 0.167µg/ml; while the Limit of Quantification (LOQ) was found to be 0.5064µg/ml for Prodrug-A.[34]
Optimized Chromatographic Conditions for Prodrug-B: HPLC system: LC 2010 CHT (Shimadzu), PDA detector (PDA-SPD-M10AVP, Shimadzu), Column (Stationary Phase): Kromasil C18 (150mm×4.6mm, 5µm particle size), Mobile phase : (Methanol: Water)(70:30 v/v), Flow Rate: 1.0 ml/min, Detection Wavelength: 262nm, Column oven Temp: 40ºC, Run time: 15 mins, Diluent: All the final dilution of sample was done with methanol.[35-37]
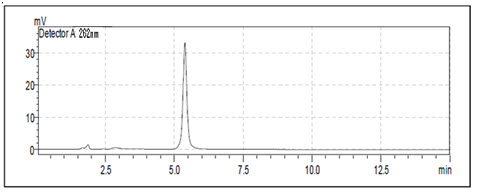
Figure-14: Chromatogram of Prodrug-B (10µg/ml) (Rt=5.5min) Prodrug-B has logP 4.9 so it releases slowly due to nonpolar nature.
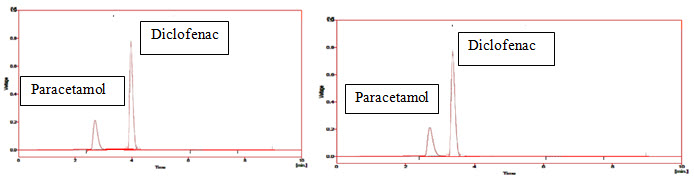
Figure-15: Chromatogram of Prodrug-B in Acidic medium (HCl: pH=3.0) and in Alkaline medium (NaOH: pH=7.5)
Paracetamol has Rt=2.75min in acidic medium and 2.8min in alkaline medium; Diclofenac has Rt=4min in acidic medium and 3.8min in alkaline medium.
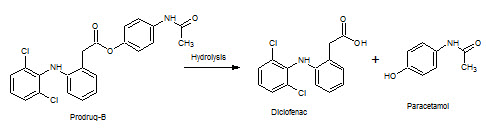
Figure-16: Prodrug–B hydrolysis into Diclofenac & Paracetamol
Method Validation:
Linearity and Range: Overlain chromatogram of Prodrug-B was shown in Figure-17. The linearity of Prodrug-B was found to be in the range of 1-25 µg/ml with correlation coefficient 0.999 as shown in Figure-18.[35]
Figure-17: Overlain Linearity Chromatogram of Prodrug-B (1-25µg/ml)
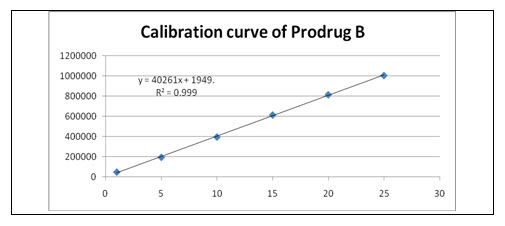 Figure-18: Calibration curve of Prodrug
Figure-18: Calibration curve of Prodrug
Precision:
1. Repeatability (Intra-day precision): The data for Intra-day precision for Prodrug-B is shown in Table-7. The %RSD for Intra-day precision was found to be 0.12-0.27% for Prodrug-B.
2.Inter-day Precision (different days): The data for Inter-day Precision for Prodrug-B is shown in Table-7. The %RSD Inter-day Precision for was found to be 0.80-1.06% for Prodrug-B.[36]
Intra-day precision data and Inter-day precision data for estimation of Prodrug-B:
|
Intra-day precision |
Conc.(µg/ml) |
Mean peak Area±S.D. |
%RSD |
Inter-day precision |
Conc.(µg/ml) |
Mean peak Area±S.D. |
%RSD |
|
10 |
396807±1078 |
0.27 |
10 |
395876±3195 |
0.80 |
||
|
15 |
613783±1209 |
0.19 |
15 |
619236±6593 |
1.06 |
||
|
20 |
814533±1005 |
0.12 |
20 |
819518±7767 |
0.94 |
*= Average of three determinations
Table-7: Intra-day precision data and Inter-day precision data for estimation of Prodrug-B
LOD and LOQ: The Limit of Detection (LOD) was found to be 0.023µg/ml; while the Limit of Quantification (LOQ) was found to be 0.071µg/ml for Prodrug-B.[37]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Optimized Chromatographic Conditions for Prodrug-C: HPLC system: LC 2010 CHT (Shimadzu), PDA detector (PDA-SPD-M10AVP, Shimadzu), Column (Stationary Phase): Kromasil C18 (150mm×4.6mm, 5µm particle size), Mobile phase : (Methanol: Water)(80:20 v/v), Flow Rate: 1.0 ml/min, Detection Wavelength: 276nm, Column oven Temp: 40ºC. Run time: 10 mins. Diluent: All the final dilution of sample was done with methanol.
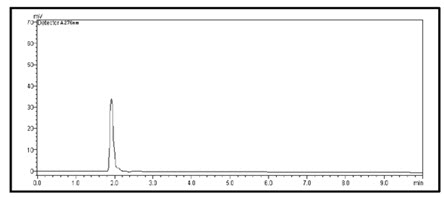
Figure-19: Chromatogram of Prodrug-C (30µg/ml) (Rt=2min)

Figure-20: Chromatogram of Prodrug-C in Acidic medium (HCl: pH=3.0) and in Alkaline medium (NaOH: pH=7.5)
Diclofenac has Rt=3.4min in acidic medium and 3.4min in alkaline medium; Ibuprofen has Rt=6.8min in acidic medium 6.35min in alkaline medium.
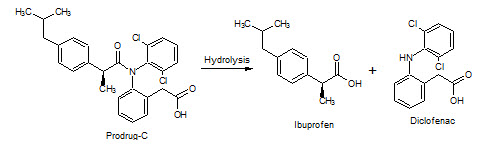
Figure-21: Prodrug–C hydrolysis into Ibuprofen & Diclofenac
Method Validation:
Linearity and Range: Overlain chromatogram of Prodrug-C was shown in Figure-22. The linearity of Prodrug-C was found to be in the range of 10-35µg/ml with correlation coefficient 0.999 as shown in Figure-23.[38]
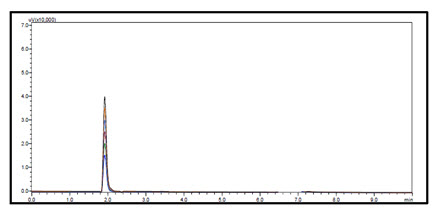
Figure-22: Overlain Linearity Chromatogram of Prodrug-C (11-35µg/ml)
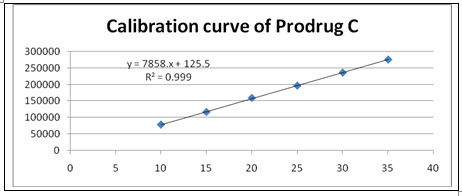
Figure-23: Calibration curve of Prodrug-C
Precision:
1. Repeatability (Intra-day precision): The data for Intra-day precision for Prodrug-C is shown in Table-8. The %RSD for Intra-day precision was found to be 0.12-0.27% for Prodrug-C.
2. Inter-day Precision (different days): The data for Inter-day Precision for Prodrug-C is shown in Table-8. The %RSD Inter-day Precision for was found to be 0.80-1.06% for Prodrug-C.[39]
Intra-day precision data and Inter-day precision data for estimation of Prodrug-C:
|
Intra-day precision |
Conc.(µg/ml) |
Mean peak Area±S.D. |
%RSD |
Inter-day precision |
Conc.(µg/ml) |
Mean peak Area±S.D. |
%RSD |
|
10 |
78678±439 |
0.55 |
10 |
78938±742 |
0.94 |
||
|
20 |
157900±1297 |
0.82 |
20 |
157234±1688 |
1.07 |
||
|
30 |
235695±1228 |
0.52 |
30 |
234695±2851 |
1.21 |
Table-8: Intra-day precision data and Inter-day precision data for estimation of Prodrug-C
LOD and LOQ: The Limit of Detection (LOD) was found to be 0.09µg/ml; while the Limit of Quantification (LOQ) was found to be 0.28µg/ml for Prodrug-C.[40]
Value of System Suitability Parameter of Prodrug-A, Prodrug-B and Prodrug-C:
|
Sr.No. |
Parameters |
Prodrug-A |
Prodrug-B |
Prodrug-C |
|
1 |
Retention time (min.) |
6.38 |
5.4 |
1.9 |
|
2 |
Theoretical plates |
17967.194 |
6696 |
2548 |
|
3 |
Tailing factor |
1.396 |
1.04 |
1.2 |
|
4 |
Resolution |
5.567 |
5.566 |
- |
|
5 |
Capacity factor |
3.3 |
2.237 |
- |
Table-9: Value of System Suitability Parameter of Prodrug-A, Prodrug-B and Prodrug-C
|
Prodrug-A |
Conc. (µg/ml) |
Peak area mean* |
SD |
%RSD |
Prodrug-B |
Conc. (µg/ml) |
Peak area mean |
SD |
%RSD |
Prodrug-C |
Conc. (µg/ml) |
Peak area mean |
SD |
%RSD |
|
20 |
561864 |
970.0 |
0.1726 |
1 |
49515 |
287 |
0.57 |
10 |
78656 |
222 |
0.28 |
|||
|
40 |
901074 |
1867.3 |
0.2068 |
5 |
195469 |
1328 |
0.67 |
15 |
116858 |
691 |
0.59 |
|||
|
60 |
1290471 |
30508.4 |
0.2383 |
10 |
395999 |
1062 |
0.26 |
20 |
159155 |
937 |
0.58 |
|||
|
80 |
1690463 |
9334.84 |
0.5527 |
15 |
613574 |
877 |
0.14 |
25 |
196324 |
1292 |
0.65 |
|||
|
100 |
2045532 |
6391.61 |
0.3127 |
20 |
813557 |
1314 |
0.16 |
30 |
235652 |
1580 |
0.67 |
|||
|
120 |
2476697 |
1051.67 |
0.0425 |
25 |
1003451 |
1297 |
0.12 |
35 |
274989 |
1483 |
0.53 |
Table-10: Linearity data of Prodrug-A, Prodrug-B and Prodrug-C
Linearity Results for Prodrug-A, Prodrug-B and Prodrug-C:
|
Regression Analysis |
Prodrug-A |
Prodrug-B |
Prodrug-C |
|
Regression equation |
Y= 19154x+15359 |
Y= 40261x+1949 |
Y= 7858x+125.5 |
|
Correlation co-efficient |
0.999 |
0.999 |
0.999 |
|
Slope |
19154 |
40261 |
7858 |
|
Intercept |
15359 |
1949 |
125.5 |
Table-11: Linearity Results for Prodrug-A, Prodrug-B and Prodrug-C
Summary of Validation Parameters for HPLC method of Prodrug-A, Prodrug-B and Prodrug-C
|
Sr.No. |
Parameters |
Prodrug-A |
Prodrug-B |
Prodrug-C |
|
|
1 |
Linearity Range |
20–120μg/mL |
1–25μg/mL |
10–35μg/mL |
|
|
2 |
Regression equation |
y=19154x+15359 |
y=40261x+1949 |
y=7858x+125.5 |
|
|
3 |
Correlation co-efficient |
0.999 |
0.999 |
0.999 |
|
|
4 |
Precision (%RSD) |
Interday |
0.17 - 0.29% |
0.80-1.06% |
0.94 - 1.21% |
|
Intraday |
0.35 - 1.50% |
0.12-0.27% |
0.52 - 0.82% |
||
|
5 |
Limit of Detection |
0.167µg/ml |
0.023µg/ml |
0.09µg/ml |
|
|
6 |
Limit of Quantification |
0.5064µg/ml |
0.071µg/ml |
0.28µg/ml |
|
Table-12: Summary of Validation Parameters for HPLC method of Prodrug-A, Prodrug-B and Prodrug-C
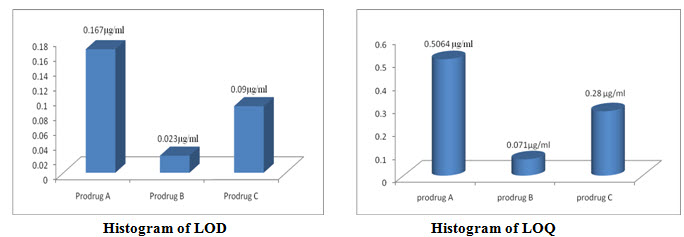
Figure-24: LOD & LOQ [Calculated by LOD=3.3 σ /s; LOQ=10 σ /s]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION:
Ibuprofen, Diclofenac & Paracetamol have been taken as NSAID and three Prodrugs have been synthesized by reacting of acid chloride of ibuprofen & diclofenac with paracetamol to get Prodrug of ester linkage and acid chloride of ibuprofen has been reacted with diclofenac to get Prodrug of amide linkage. Prodrug is a substance which after administration is metabolized into a pharmacologically active drug. Actually Prodrug has least medicinal value in in-vitro/in-vivo but after biotransformation by metabolism in in-vivo it releases the active medicament. A drug is a substance which is a chemical entity, has definite structural skeleton, obtained by natural or synthetic or semisynthetic source, which can fit on bioreceptor platform having controlling capacity to control over the biochemical malfunction. Every drug is xenobiotic because it is coming from outer source (xeno) and active in biological unit (biotic). Prodrug is the precursor of drug which is made by derivatization of the same to enhance the bioavailability by pharmacokinetics, lipid solubility by partition coefficient and increase the physicochemical & biochemical parameters by pharmacodynamics. All three Prodrugs showed different logP values and molecular weights according to the solubility parameters and electronegativity: logP profile: Prodrug-C>Prodrug-B>Prodrug-A; molecular weight profile: Prodrug-C>Prodrug-B>Prodrug-A.
The main side effect of NSAID is gastric acidity due to release of free H+ because all NSAIDs have free –COOH (carboxylic acid) group which act by competitive inhibition of cyclooxygenase enzyme (COX1/COX2). Here the target of this project has been designed in such a way to convert the free –COOH of API (ibuprofen/diclofenac/paracetamol) into Prodrug of ester (–COO–) as well as amide (–CONH–) linkage (Prodrug-A/Prodrug-B/Prodrug-C) which releases free API after metabolic hydrolysis in acidic pH: 1-4 and alkaline pH: 7-9. Since the Prodrugs are repository forms so chances to release gastric acid has been minimised due to non availability of free –COOH group in stomach. The biotransformation of active drug from Prodrug takes such a time in stomach that all goes upto duodenum and then ileum of small intestine that chances of acidity is reduced. Finally all Prodrugs go to small intestine where alkaline pH starts so gastric acidity is reduced. Since all three Prodrugs are made of two NSAID: Prodrug–A (logP=4.56) releases Ibuprofen & Paracetamol, Prodrug–B (logP=4.90) releases Diclofenac sodium & Paracetamol and Prodrug–C (logP=6.13) releases Ibuprofen & Diclofenac which shows distinct two Rt values in HPLC both in acidic an alkaline hydrolysis and these Rt values of Prodrugs match with the individual API components so the purpose of our goal has been completed successfully. The pH of gastric acid varies from 1.5-3.5 in the human stomach lumen, the acidity being maintained by the proton pump H+/K+ ATPase. So the pattern for acid hydrolysis was adjusted at pH=3-3.5 by HCl. The pH of intestine varies from 5.6-6.9, so the pattern for alkaline hydrolysis was adjusted at pH=7.0-8.0 by NaOH.
This project has been divided into three parts: 1. Synthesis of Prodrug of ester and amide linkages of NSAID having carboxylic acid, phenolic and imino groups [http://www.wjpps.com/wjpps_controller/abstract_id/6123]
2. Logarithmic partition coefficient comparison study and molecular weight of synthesized Prodrugs of ibuprofen+paracetamol, diclofenac sodium+paracetamol and ibuprofen+diclofenac sodium [http://www.imedpub.com/articles/logarithmic-partition-coefficient-comparisonstudy-and-molecular-weight-of-synthesizedProdrugs-of-ibuprofenparacetamol-diclofenacso.pdf]
3. Study of in-vitro biotransformation of Prodrugs of ester and amide linkages of ibuprofen, diclofenac sodium and paracetamol in acidic and alkaline medium.
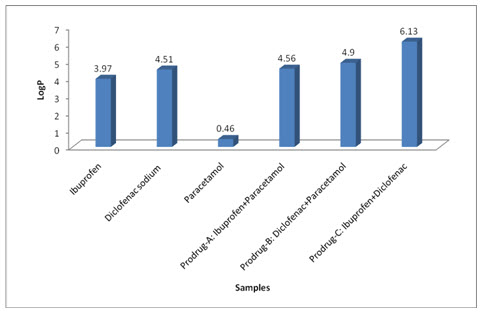
Standard Rt of NSAIDs:
1. Paracetamol releases first (Rt=2.3min) because it’s logP is 0.46 (highly polar). 2. Ibuprofen releases slow (Rt=6.8min) because it’s logP is 3.97 (semipolar). 3. Diclofenac sodium releases moderate (Rt=3.4min) because it’s logP is 4.51 (nonpolar).
Prodrug Rt after hydrolysis:
Prodrug-A [-COO-; ester linkage]: Paracetamol has Rt=2.5min in acidic medium and 3.5min in alkaline medium; Ibuprofen has Rt=6.35min in acidic medium and 6.15min in alkaline medium.
Prodrug-B [-COO-; ester linkage]: Paracetamol has Rt=2.75min in acidic medium and 2.8min in alkaline medium; Diclofenac has Rt=4min in acidic medium and 3.8min in alkaline medium.
Prodrug-C [-CONH-; amide linkage]: Diclofenac has Rt=3.4min in acidic medium and 3.4min in alkaline medium; Ibuprofen has Rt=6.8min in acidic medium 6.35min in alkaline medium.
Rt of paracetamol varies between 2.5-3.5min; Rt of ibuprofen varies between 6.15-6.8min and Rt of diclofenac varies between 3.4-3.8min. The focus of in-vivo metabolic profile of Prodrugs has been implemented into in-vitro hydrolytic reaction in both acidic and alkaline pH to get the satisfactory desired result
REFERENCES:
1. Jalpa G. Patel and Prof. Dr. Dhrubo Jyoti Sen; Synthesis of Prodrug of ester and amide linkages of NSAID having carboxylic acid, phenolic and imino groups: World Journal of Pharmacy and Pharmaceutical Sciences; 2016; 5(11): 897-908.
2. Dhrubo Jyoti Sen and Jalpa G. Patel; Logarithmic partition coefficient comparison study and molecular weight of synthesized Prodrugs of ibuprofen+paracetamol, diclofenac sodium+paracetamol and ibuprofen+diclofenac sodium: American Journal of Advanced Drug Delivery: 2016, 4(05), 064-068.
3. J.E. Slemmer, B.R. Martin and M.I. Damaj. Bupropion is a nicotinic antagonist. J. Pharm. Exp. Ther., 2000; 295: 321–327.
4. C. Wu, J. Quan, J. Xie, C Branford-White, L. Zhu, Y. Yu and Y. Wang. Preparation and controlled release of degradable polymeric ketoprofen-saccharide conjugates. Polym Bull., 2011; 67: 593–608.
5. M, Babazadeh and T. Mosanejhad, Vinyl ester type polymers containing ibuprofen pendents: synthesis, characterization and evaluation. Iran Polym J., 2009; 18: 179–186.
6. L. Shargel, Susanna Wu-Pong and B.C. Andrew, Applied Biopharmaceutics & Pharmacokinetics, 6th Ed.McGraw-Hill Medical Publishing Division, US., 2012; 47-66, 129-154.
7. S. Shirke*, S. Shewale and M. Satpute, Prodrug design: an overview. International Journal of Pharmaceutical, Chemical and Biological Sciences., 2015; 5(1): 232-241.
8. A.V. Bhosale, G.P. Agrawal and P.Mishra: Preparation and Evaluation of Directly Compressible Forms of Mutual Prodrugs of Ibuprofen Forms of Mutual Prodrugs of Ibuprofen. Indian Journal of Pharmaceutical Sciences, 2006; 68(4): 425-431.
9. Beckett AH and Stenlake JB, Practice pharmaceutical chemistry; 4th Edn; CBS Publishers and Distributors, New Delhi, 1997, 293-304.
10. Ewing GW, Instrumental Methods of Chemical Analysis; 5th Edn; McGraw- Hill Book Company, New York, 1985, 1-7.
11. Kasture AV, Mahadik KR, Wadodkar SG and More HN, Instrumental methods of pharmaceutical analysis; 14th Edn; Nirali Prakashan, Pune, 2006, 1-30.
12. Wikipedia: The free Encyclopedia, “Ultraviolet-visible spectroscopy”, 2013, http://en.wikipedia.org/wiki/Ultraviolet-visible spectroscopy.
13. Davidson AG, Practical Pharmaceutical Chemistry; 4th Edn; CBS Publishers and Distributors, 1997, 275-337.
14. Skoog DA, Holler FJ and Nieman TA, Principle of Instrumentation Analysis; 5th Edn; Thomas Asia Pvt Ltd, 2005, 580.
15. Sharma BK, Instrumental Method of Chemical Analysis; 25th Edn; Krishna Prakashan Media Ltd, 2006, 183-186.
16. Beckett AH and Stenlake JB, Practical Pharmaceutical Chemistry; 4th Edn; CBC Publication and distribution, 1977, 1-26.
17. Snyder LR., Kirkland JL and Glajch JL., Practical HPLC Method Development.,2nd Edition, Wiley Interscience, 1977, 1- 26.
18. Halmilton RJ and Sewell PA., Introduction to HPLC., 2nd Edition; Chapman and Hall, 1982, 189.
19. Stability Testing of New Drugs and Product: ICH Q1 A (R2), 2003, 1- 20.
20. Cartensen JT and Rhodes CT., Drug stability: Principles and practices New York – Marcel Dekker; 3rd Edition, 329 – 384.
21. Riley CM and Rosanske TW., Progress in Pharmaceutical and Biomedical Analysis., Development and validation of analytical methods., 1st Edition ; Elsevier Science Ltd, USA, 1996, 133–135.
22. Haynes, W.M. (ed.). CRC Handbook of Chemistry and Physics. 94th Edition. CRC Press LLC, Boca Raton: FL 2013-2014, p. 3-314
23. Yalkowsky, S.H., He, Yan, Jain, P. Handbook of Aqueous Solubility Data Second Edition. CRC Press, Boca Raton, FL 2010, 492.
24. O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013, 10.
25. American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, 2212.
26. O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001, 876.
27. Yalkowsky SH, Dannenfelser RM; The AQUASOL database of Aqueous Solubility. Ver 5. Tucson, AZ: Univ AZ, College of Pharmacy, 1992.
28. Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980, 1057.
29. Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996, 712.
30. Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976, 22.
31. O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006, 522.
32. Finn A et al; Acta Technol Legis Med, 1986, 4: 33-44.
33. American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, 2113.
34. Anojo Hetal; Eur J Drug Metab Pharmacokinetic, 1999, 24(4): 345-51.
35. Hosale AV, Agrawal GP and Mishra P: Preparation and characterization of mutual Prodrugs of ibuprofen. IJPSR 2004; Vol. 2, Issue 4 ISSN: 0975-8232.
36. Koren, K., Mann, A.R., Colosimo, A.L., Diaz, Z., Nuclelman, A., Levovich, I., Jing, Y., Waxman, S. and Miller, W.H. Jr., Mol. Cancer Res., 2003, 1, 903.
37. Slemmer J.E., Martin B.R., Damaj M.I. Bupropion is a nicotinic antagonist. J. Pharm. Exp. Ther. 2000; 295: 321–327.
38. Wu C, Quan J, Xie J, Branford-White C, Zhu L, Yu Y, Wang Y. Preparation and controlled release of degradable polymeric ketoprofen-saccharide conjugates. Polym Bull. 2011; 67: 593–608.
39. Gennaro, R., Alfanso, W., Remington: The science and practice of pharmacy, Lippincott Williams & Wilkins, 2003, 20, 1, 913-914.
40. Shargel AB and Andrew BC, Applied Biopharmaceutics & Pharmacokinetics, 4th Ed. McGraw-Hill Medical Publishing Division, US. 47-66, 129-154.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









