About Authors:
Ms.Pratibha Chohan*, Mr.Prashant Mutha
B.Pharmacy, G. D. Memorial College of Pharmacy, Jodhpur.
*pmuthabiotech@gmail.com
ABSTRACT:
Brain is tightly segregated from the circulating blood by a unique membranous barrier, the blood – brain barrier (BBB). Many pharmaceuticals cannot be efficiently delivered to, or sustained within the brain; hence they are ineffective in treating a plethora of cerebral diseases. Therefore, drug delivery methods that can provide drug delivery to brain or eventually preferential brain delivery (i.e. brain targeting) are of particular interest. One technique that holds promise for bypassing the BBB to deliver drugs to the brain and eliminating the surgical risk and the spillover effect of drug to normal tissue is the intranasal delivery.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1440
Introduction
The connection of nasal cavity to brain tissue is known to mankind from the time which stands centuries back . The Indian system of medicine, Ayurveda, clearly describes the administration of several drugs intended to be delivered to brain via the nasal cavity. There are many practical examples cited in number of old texts which endorses the facts. In the present context pertaining to relevance and space, there is a special method called “Sneha - Paka - Widhi” described in Ayurveda. This process bypasses the Blood Brain Barrier and allows the active herbal constituents to reach the central nervous system and arrest the progress of disesases of Central Nervous System eventually. Besides this, the nasal medication also helps to improve vision, arrest hair fall, prevent graying of hair, improve hearing and many other diseases attributed to ENT and supra-clavicular disorders. Like synthetic drugs have side effects, Ayurvedic drugs have side benefits. Unknowingly these formulations impart some added benefits and contribute to positive health achievements.As contemporary era of biotechnology continues to advance, nasal drug delivery is increasingly becoming a more viable alternative to oral and injectable routes of administration. The nasal route could be particularly important for drugs used in crisis management such as for pain and for centrally acting drugs where the pathway from the nose to brain might provide a faster and more therapeutic effectIn recent years many drugs have been shown to achieve better systemic bioavailability through nasal route than by oral administration. Advances in biotechnology have made available a large number of protein and peptide drug for the treatment of a variety of diseases.
[adsense:468x15:2204050025]
The nasal mucosa has been considered as a potential administration route to achieve faster and higher level of drug absorption. This is due to the large surface area, porous endothelial membrane, high total blood flow, the avoidance of first-pass metabolism, and ready accessibility. The nasal administration of drugs, including numerous compounds, peptide and protein drugs, for systemic medication has been widely investigated in recent years. In the recent past many researchers have also attempted delivery of drugs to the CNS through the nose. However, the major limitation with nasal route administration is the poor contact of the formulations with the nasal mucosa. Many attempts have been made in the recent past to increase the residence time of drug formulations in the nasal cavity, resulting in improved nasal drug absorption. Researchers became interested in the nasal route for the systemic delivery of medication due to high degree.(1)
Blood-Brain Barrier
The blood-brain barrier is a dynamic barrier that separates the systemic circulation from the brain. The BBB not only impedes brain penetration of some substances, but it also actively transports necessary nutrients and electrolytes from the systemic circulation to the brain. The BBB is comprised of capillaries with a single layer of endothelial cells, parricides, the basal lamina, and atrocity projections (Hawkins and Davis, 2005). It has been estimated that there are >100 billion capillaries in the human brain, separated by a distance of approximately 50 μm (Partridge, 2005). As a result of this small distance between brain capillaries, it has been hypothesized that each neuron is perfuse by its own blood capillary (Pardridge, 2005). Furthermore, this translates to a molecular diffusion distance of 25 μm within brain parenchyma following intravascular administration of drugs that either penetrate the BBB, or bypass the BBB asis the case for intranasal administration (Pardridge, 2005).
Transport Across the Blood-Brain Barrier
Several different techniques have been attempted to deliver drugs and therapeutic proteins across the BBB and into the brain. In order to understand these techniques and how to best employ them, we must first understand the main transport mechanisms that naturally exist in the blood-brain barrier. There are six main transport mechanisms : par cellular, transcellular, facilitated transport, receptor mediated endocytosis, adsorptive endocytosis, and carrier mediated efflux transport (Figure No. 1) (Neuwelt, 2004). Para cellular transport involves the movement of very small water soluble molecules, down their concentration gradient, through the tight junctions of cerebral endothelial cells (Neuwelt, 2004). Tran cellular transport, on the other hand, involves the movement of low molecular weight, highly lipophilic substances across the plasma membranes of endothelial cells via passive diffusion (Neuwelt, 2004). Facilitated Transport, or carrier mediated transport, is an energy independent mechanism that employs membrane bound proteins that undergo conformational changes, and transport Substances down their concentration gradient (Neuwelt, 2004)(2).
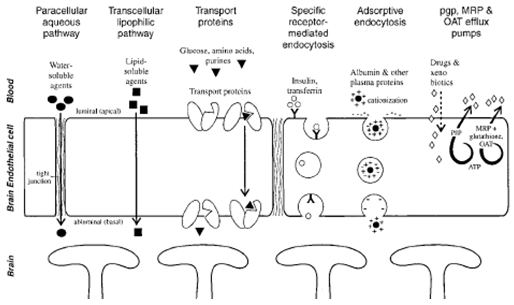
Fig.No.1
Drug Delivery across the Blood-Brain Barrier
Several techniques have been utilized to deliver therapeutic moieties across the blood-brain barrier. The four main approaches are: BBB disruption, bypassing the BBB, using chimerical translocation proteins, and using micro particles to deliver drugs to the brain. BBB disruption takes advantage of the anatomical characteristics of tight junctions, and attempts to increase par cellular (or in between cells) transport by interfering with tight junction formation and/or integrity. However, long-term disruptions of the BBB could potentially result in the passage of toxic substances and/or pathogens into the CNS. Broman and Olsson, in 1940, were the first to attempt to deliver a substance to the brain by disrupting the BBB by using contrast dyes (Kroll and Newel, 1998). However, it was Rapport and colleagues who first proposed using hyperosmolar solutions to increase the delivery of substances to the brain (Kroll and Newel, 1998). Hyperosmolar solutions are believed to cause a disruption of the BBB by causing endothelial cell dehydration and shrinking, thereby loosening tight junctions and increasing intercellular space. The most commonly used hyper osmotic agent for BBB disruption is intravenous mannitol because it is FDA approved, safe, effective, inexpensive, and its effects are temporary. However, agents that disrupt the integrity of the BBB are non-specific, and can also increase BBB permeability to toxins and pathogens making this approach a less attractive alternative. Bypassing the BBB is another method that has been employed in order to deliver drugs to the brain. The presence of an incomplete BBB in the nasal mucosa has led to attempts to deliver drugs via the intranasal route of administration. An extensive discussion on this subject will follow. Chimerical peptide technology has also been utilized to improve BBB permeability.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Brain Transport and the Intranasal Route of Administration
In recent years, the intranasal route of administration has emerged as an attractive method for delivering brain impermeable drugs and proteins to the CNS. This is because intranasal drug administration is generally well tolerated, non-invasive, and because the olfactory route of administration completely bypasses the blood brain barrier. As a result, drugs and/or proteins can be transported directly from the nasal epithelium into the brain (Graff and Pollack, 2005). Due to their functions of smell and sensory perception, the olfactory and trigeminal neurons’ nerve terminals are exposed to the nasal cavity, and serve as the interface between the outside world and the brain. In addition, there is an anatomical connection between the olfactory system and the midbrain (Figure No. 2). Brain regions involved in olfactory perception send axonal projections directly to the limbic areas of the brain.
This anatomical connection between the midbrain and the olfactory system may become a major advantage in the treatment of PD by providing a direct access for drugs (and/or therapeutic proteins) to the areas of the brain most devastated by PD.
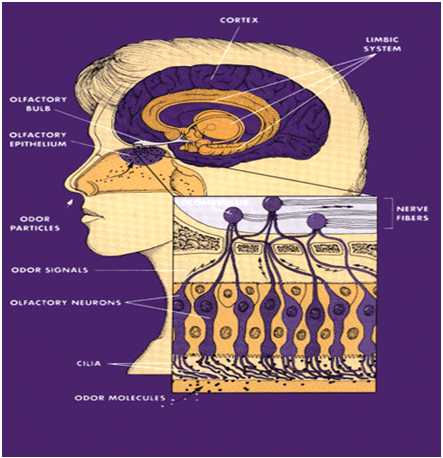
Fig.No.2
In order for drug uptake to occur in the nasal cavity, drugs must first come into contact with the nasal epithelium, which is constantly covered by a protective mucus layer. This layer of mucus is recycled every 10-15 minutes, removing all particulate matter (including drugs) to the back of the throat for elimination (Talegaonkar and Mishap, 2004). Therefore, it is crucial to maximize residence time (how long the drug or protein is present) in the nasal cavity.
Once through the mucus layer, there are 3 main mechanisms by which drugs undergo nose-to-brain transport: transcellular, par cellular (Figure No.3) and axonal transport (Illum, 2003). Tran cellular (or across cells) drug transport involves endocytosis through the Olfactory epithelial cells, which surround the olfactory neurons. Tran cellular transport can be either receptor mediated, or through passive diffusion, and results in both brain and systemic transport (Illum, 2003). Lipophilic, low molecular weight (MW) molecules typically undergo transcellular transport. For example, fentanyl, Which is very lipophilic, is 80% bioavailable when administered intranasal (Illum, 2003). On the other hand, small polar molecules are only 10% bioavailable, and peptides are less than 1% bioavailable when administered intranasally (Illum, 2003). Transport of small lipophilic drugs via this route is very fast; it occurs within minutes (Frey 2002).
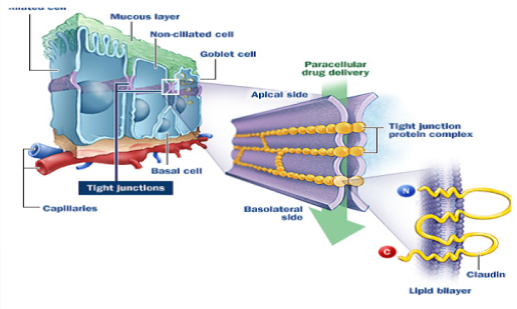
Fig. No. 3 : Paracellular transport of xenobiotics through the tight junctions of the olfactory epithelium.
The use of intranasal (IN) administration to target therapeutics to the central nervous system (CNS) has many benefits in the treatment of neurologic disorders. The blood-brain barrier (BBB) restricts the use of numerous therapeutic agents that have been developed to treat memory loss and neurodegeneration because it limits CNS penetration, depending on drug size or charge. Although invasive methods of administration (for instance intracerebroventricular) have been used to overcome the BBB, these methods are not practical for use in humans for several reasons, including convenience, safety, and cost. Direct delivery of therapeutics from the nasal cavity into the CNS (IN delivery) bypasses the BBB and provides an alternative to invasive methods of drug administration . Noninvasive IN delivery targets therapeutics to the CNS, reducing systemic exposure and side effects; this can be advantageous for delivery of many CNS therapeutics, including those that can cross the BBB upon systemic administration. CNS therapeutics do not necessarily need to be modified for IN delivery, and delivery of therapeutics to the CNS is rapid, occurring within minutes. The IN delivery method was first developed by Frey in 1989 for targeting neurotrophic factors (for example, nerve growth factor [NGF] and fibroblast growth factor-2) to the CNS(3).
Olfactory and trigeminal pathway
Intranasal administered therapeutics reach the CNS via the olfactory and trigeminal neural pathways. Both the olfactory and trigeminal nerves innervate the nasal cavity, providing a direct connection with the CNS. Direct delivery of therapeutics from the nose to the brain was initially attributed to the olfactory pathway . More recently, the contribution made by the trigeminal pathway to IN delivery to the CNS has also been recognized, especially to caudal brain regions and the spinal cord . Extracellular delivery, rather than axonal transport, is strongly indicated by the short time frame (≤ 10 minutes) observed for IN therapeutics to reach the brain from the nasal mucosa. Possible mechanisms of transport may involve bulk flow and diffusion within per neuronal channels, per vascular spaces, or lymphatic channels directly connected to brain tissue or cerebrospinal fluid .
General principlesof intranasal medication delivery
The nasal cavity's easily accessible, rich vascular plexus permits topically administered drugs to rapidly achieve effective blood levels while avoiding intravenous catheters. This is most effectively accomplished by distributing drug solutions as a mist rather than as larger droplets which may aggregate and run off instead of being absorbed. Because of this easily accessed vascular bed, nasal administration of medications is emerging as a promising method of delivering medications directly to the blood stream ( Figure No. 4 ) . This method of delivery can eliminate the need for intravenous catheters while still achieving rapid, effective blood levels of the medication administered.
Fig. No. : 4
Administering medications via the nasal mucosal offers several advantages:
1. The rich vascular plexus of the nasal cavity provides a direct route into the blood stream for medications that easily cross mucous membranes.
2. This direct absorption into the blood stream avoids gastrointestinal destruction and hepatic first pass metabolism (destruction of drugs by liver enzymes) allowing more drug to be cost-effectively, rapidly, and predictably bioavailable than if it were administered orally.
3. For many IN medications the rates of absorption and plasma concentrations are comparable to intravenous administration, and are typically better than subcutaneous or intramuscular routes.
4. Ease, convenience and safety: IN drug administration is essentially painless, and does not require sterile technique, intravenous catheters or other invasive devices, and it is immediately and readily available for all patients.
5. Because the nasal mucosa is nearby the brain, cerebrospinal fluid (CSF) drug concentrations can exceed plasma concentrations. IN administration may rapidly achieve therapeutic brain and spinal cord (CNS) drug concentrations.
6. Studies in cynomolgus monkeys have demonstrated intranasal delivery bypasses the BBB to deliver interferon beta-1b to the CNS (Thorne et al., 2007 in press) and delivers a prostaglandin analog to the brain to induce sleep in these primates (Yamada et al., 2007)Born et al. (2002) have indicated that intranasally administered neuropeptides such as melanocortin, insulin, and vasopressin gained access to the CSF in humans in as little as 10 minutes
7. Human studies have demonstrated that intranasal insulin enhances memory and mood in healthy adults (Benedict et al., 2004). Intranasal insulin aspart was shown to be even more effective than regular insulin or placebo in improving memory in normal human adults with no change in the blood levels of insulin or glucose (Benedict et al., 2007). In addition, intranasal insulin improves memory in patients with Alzheimer's disease without affecting insulin or glucose blood levels (Reger et al., 2006). Craft et al. have presented some data at the 10thInternational Conference on Alzheimer’s disease (ICAD 2006) indicating that administration of intranasal insulin for twenty-one days significantly improved verbal memory in Alzheimer's patients. Moreover, Intranasal insulin has been demonstrated to reduce body fat in men (Hallschmid et al., 2004).Other studies have shown that intranasal PT-141 (melanocortin analog) enhances erection in humans with erectile dysfunction by acting at the hypothalamic melanocortin receptors implicated in both appetite and sexual response (Diamond et al., 2004). Direct targeting of the neuropeptide oxytocin from the nose to the brain has been found to substantially increase trust in humans (Kosfeld et al., 2005).Gene therapy of the CNS can be administered through the nasal route. Intranasal delivery of adenoviral vectors and plasmid DNA to the brain has been documented (Draghia et al., 1995; Han et al., 2007). Finally, recent studies have established that intranasal delivery of the recombinant strain of the bacterium Lactococcus lactis secreting leptin has led to significant reduction of food intake and body weight (Bermúdez-Humarán et al., 2007).
Intranasal delivery of drugs to brain
There are many classes of medications that may be used intranasally, many of which are applicable to the pre-hospital and emergency setting. These medications include antiepileptics, opiate analgesics and opiate antagonists, sedatives, topical anesthetics, glucagon for hypoglycemia, and agents for epistaxis control.(4)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Opioid Peptides Delivery to Brain
Peptide drug delivery to the brain may be divided into 3 general categories:
a. Invasive procedures, which include transient osmotic opening of the BBB, 73 , 74 shunts, 75 and biodegradable implants. 76 , 77 These procedures can be highly traumatic and often have low therapeutic effigy cadency with substantial side effects.
b. Pharmacologically-based approaches to increase the passage through the BBB by increasing specific biochemical attributes of a compound. This may be accomplished either by chemical modification of the peptide molecule itself, or by the attachment or encapsulation of the peptide in a substance that increases permeability, stability, bioavailability, and/or receptor affinity. In addition, modification of drug structure and/or addition of constituents (eg, lipophilicity enhancers, polymers, antibodies) may enhance drug concentration within the CNS, with a reduced toxic profile.
c. Physiologic-based strategies exploit the various carrier mechanisms at the BBB, which have been characterized in recent years for nutrients, peptide and no peptide hormones, and transport proteins.
Intranasal delivery of stem cells to the brain
Stem cell-based therapy has proved to be a promising treatment option for neurological disorders. However, there are difficulties in successfully administrating these stem cells. For example, the brain-blood barrier impedes the entrance of stem cells into the CNS after systemic administration. Direct transplantation or injection may result in brain injury, and these strategies are clinically less feasible. Intranasal administration is a non-invasive and effective alternative for the delivery of drugs, vector-encoded viruses or even phages to the CNS. Recent studies have in fact demonstrated that stem cells may enter the CNS after intranasal administration. These results suggest that intranasal delivery may provide an alternative strategy for stem cell-based therapy. Areas covered. Intranasal delivery of stem cells is a new method with great potential for the transplantation of stem cells into the brain, and it may provide an extraordinary approach to overcoming the existing barriers of stem cell delivery for the treatment of many neurological disorders. This potential benefit emphasizes the importance of future research into intranasal delivery of stem cells.(5)
a. Safe delivery of stem cells in Parkinson’s Disease.
The international team of scientists found that intranasal application of mesenchymal stem cells to the brains of rats with Parkinson’s disease resulted in the improvement in animals’ motor function and higher levels of neurotransmitter dopamine. This procedure may be a noninvasive alternative to the traumatic surgical transplantation of stem cells to the brain which is commonly used for therapy of neurodegenerative disorders, including Parkinson’s treatment. The drops containing bone marrow stem cells were delivered intranasal to the rats with Parkinson’s disease. The highest percentage of these cells was observed in cortex, cerebellum, brainstem, and spinal cord 4 hours after application. It proved that stem cells migrate rapidly along the nerves to the brain. These delivery routes are also known to be involved in the intranasal delivery of drugs to the brain.
The research demonstrated that stem cells survived in the rat brains for a period of at least 6 months. Their application increased the levels of the dopamine. Besides, the stem cells showed strong anti-inflammatory and neuroprotective effect to the brain. The behavioural tests revealed significant improvement of motor function of the Parkinsonian rats up to 68% of the normal valuein40-110days after stem cell delivery.
Thus, intranasal administration of stem cells provides new effective approach to the Parkinson’s treatment. Moreover, it can be useful method for treating other pathologies such as amyotrophic lateral sclerosis (ALS) and multiple sclerosis. This non-invasive treatment may be used repeatedly increasing the number of delivered cells to the brain which enhance the therapeutic effect. (6)
b. Intranasal delivery of telomerase inhibitor GRN163.
The blood-brain barrier is a substantial obstacle for delivering anticancer agents to brain tumors, and new strategies for bypassing it are greatly needed for brain-tumor therapy. Intranasal delivery provides a practical, noninvasive method for delivering therapeutic agents to the brain and could provide an alternative to intravenous injection and convection-enhanced delivery. Despite the development of drugs that preferentially target tumor cells without harming normal tissues, delivery of these drugs to brain tumors remains a major challenge because of difficulty in penetrating the blood-brain barrier (BBB). Malignant gliomas are the most common primary tumors that occur in the human brain. The 5-year survival rate for patients with glioblastoma (GBM), the most aggressive form of malignant glioma, is less than 5% even with surgery followed by radiation therapy and chemotherapy. Clearly there is a great need for new therapeutic strategies that will provide efficient drug delivery to the brain tumors. Intranasal delivery provides a practical, noninvasive method for delivering therapeutic agents to the brain because of the unique anatomic connection provided by the olfactory and trigeminal nerves. These nerves connect the nasal mucosa and the CNS, allowing them to detect odors and other chemical stimuli. Intranasally administered drugs reach the parenchymal tissues of the brain and spinal cord and/or cerebrospinal fluid (CSF) within minutes using an extracellular route through perineural channels. In addition to bypassing the BBB, the advantages of intranasal delivery include rapid delivery to the CNS, avoidance of hepatic first-pass drug metabolism, and elimination of the need for systemic delivery, thereby reducing unwanted systemic side effects. Intranasal delivery also provides painless and convenient self-administration by patients, features that encourage its use for delivering therapeutic agents into the CNS.
The safety and efficacy of cell-based therapies for neurodegenerative diseases depends on the mode of cell administration. We hypothesized that intranasally administered cells could bypass the blood-brain barrier by migrating from the nasal mucosa through the cribriform plate along the olfactory neural pathway into the brain and cerebrospinal fluid (CSF). This would minimize or eliminate the distribution of cellular grafts to peripheral organs and will help to dispense with neurosurgical cell implantation. Here we demonstrate transnasal delivery of cells to the brain following intranasal application of fluorescently labeled rat mesenchymal stem cells (MSC) or human glioma cells to naive mice and rats. After cells crossed the cribriform plate, two migration routes were identified: (1) migration into the olfactory bulb and to other parts of the brain; (2) entry into the CSF with movement along the surface of the cortex followed by entrance into the brain parenchyma. The delivery of cells was enhanced by hyaluronidase treatment applied intranasally 30 min prior to the application of cells. Intranasal delivery provides a new non-invasive method for cell delivery to the CNS.(6)
Cell-based therapy is one of the future therapeutic strategies for neurodegenerative diseases. A big challenge is the method of cell delivery which may influence the graft survival, sufficient enrichment of therapeutic cells in the brain and avoidance of their distribution throughout the peripheral organs. We hypothesized that eukaryotic cells may bypass the blood-brain-barrier by migrating from the nasal mucosa into the brain. This hypothesis was tested by intranasal (IN) application of mesenchymal stem cells and glioma cells in young and adult mice and rats. Both cell types reached the olfactory bulb (OB) after IN-application and were found in other areas of the brain (cortex, hippocampus, striatum and cerebellum). After cells crossed the cribriform plate, two migration routes were observed: 1) migration through the OB to the hind parts of the brain; 2) entry into the CSF followed by entrance into the parenchyma from the cortex. Cell delivery to the brain was enhanced by pre-treatment of the nasal mucosa with hyaluronidase. (7)
c. Intranasal Stem Cells Improve Motor Function in Parkinson's
Research regarding neural (nerve) cell transplantation as a possible treatment for Parkinson’s disease has been underway since the early 1990s. That’s when scientists transplanted neurons from the human embryonic brain into the brain of patients who had Parkinson’s and saw long-lasting therapeutic benefits. Subsequent studies using this approach, however, were mostly negative, and some patients developed involuntary movements, termed graft-induced dyskinesias, as side effects. Although research in the area of neural cell transplantation continues, the potential for stem cell transplantation has grown.William H. Frey II, PhD, director of the Alzheimer’s Research Center, and Lusine Danielyan, MD, of University Hospital of Tubingen in Germany, reported on the results of their latest work using stem cells to treat Parkinson’s disease. The scientists used a rat model of the disease to show that many of the stem cells they delivered intranasally survived for at least six months in the brain, and that the stem cells rapidly moved to damaged areas of the brain. Motor function in the treated rats also improved.
The positive effects seen in this study are believed to have occurred because the stem cells, which were harvested from bone marrow, produced anti-inflammatory and neuroprotective effects in the damaged brain regions. In 2009, scientists from the University of California, Los Angeles, reported on the world’s first clinical trial using autologous (derived from the patient) neural stem cells for treatment of Parkinson’s. Although only one patient was treated, the authors reported that the individual’s motor scales improved by more than 80 percent for at least 36 months. Current treatment options include medications, surgery, and deep brain stimulation. The intranasal delivery of stem cells to treat Parkinson’s disease may be a promising alternative for patients who have this debilitating disease that severely compromises motor function and quality of life. Additional research will explore how to increase the number of stem cells delivered to the brain and improve the benefits of such treatment.(8)(9)
Future Prospects:
Cumulating evidences have suggested that intranasal delivery of Stem cells, Peptides and drugs through the Olfactory Pathways and Trigeminal nerves is possible. Intranasal delivery has multiple significant advantages over the conventional rouites as both drugs and cells can be transported into the brain by bypassing the BBB, potential side effects on the peripheral system can be eradicated,the non invasive process reduces the pain and trauma to the patients. What remains still of interest is the answers to many queries. The future studies of interest can be, Further investigation of intranasal route delivery to brain mechanism at human level in particular, conduct of clinical trials in Neurological disorders and stroke which may lead to a fundamental breakthrough in therapeutic strategies for the diseases.(10)
REFERENCES:
1. ayurdoctor.com/Article10.asp
2. Gowda D.V,* Tanuja. D, Mohammed S. Khan, Desai. J, Shivakumar H.G, Scholars Research Library Der Pharmacia Lettre, 2011, 3(1): 371-381
3. Mattia M. Migliore “Intranasal delivery of GDNF for the treatment of Parkinson's disease” Pharmaceutical Science Dissertations January 01, 2008
4. neuralrepairinstitute.org/intranasaldelivery.html
5. Shyeilla V. Dhuria, PhD, Leah R. Hanson, PhD, and William H. Frey II “FORMULATION - Drug Delivery | Intranasal Delivery to the Central Nervous System” Journal of Pharmaceutical Sciences (Vol. 9, No. 4; pp. 1654-1673)
6. springerlink.com/content/w257l334311275u1/
7. Leah R Hanson and William H Frey II “Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat” BMC Neuroscience 2008, 9(Suppl 3):S5
8. emaxhealth.com/1275/intranasal-stem-cells-improve-motor-function-parkinsons
9. ncbi.nlm.nih.gov/pubmed/19332330
10. Paul A Lapchack,”Translational Stroke Research: From target selection to clinical trials”Springer,Page No.686,687.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









