About Authors:
Kalpesh N. Patel1*, Jayvadan K. Patel2
1 Research Scholar, Jodhpur National University, Jodhpur,
Rajshathan, India
2 Department of Pharmaceutical Technology,
Nootan Pharmacy College,
Visnagar (GUJARAT), India.
Abstract
Two simple spectrophotometric methods have been developed for simultaneous estimation of pyrimethamine and sulphadoxine from tablet dosage form. Method-I simultaneous equation method involves the measurement of absorbances at two wavelengths 215 nm (λmax of pyrimethamine) and 254 nm (λmax of sulphadoxine) in methanol and Method-II first order derivative spectroscopic method involves the measurement of absorbances at two wavelengths 250 nm (λmax of pyrimethamine) and 220.5 nm (λmax of sulphadoxine); The linearity lies between 5-30 µg/ml for both pyrimethamine and sulphadoxine for all the two methods. The accuracy and precision of the methods were determined and validated stastically. All the methods showed good reproducibility and recovery with % RSD less than 2. All method were found to be rapid, specific, precise and accurate and can be successfully applied for the routine analysis of pyrimethamine and sulphadoxine in bulk and combined dosage form.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1228
INTRODUCTION
Chemically, Pyrimethamine (PYR) is 5-(4-chlorophenyl)-6-ethyl-2,4-pyrimidinediamine[1].Itis a medication used for protozoal infections. It is commonly used as an antimalarial drug and is also used in the treatment of Toxoplasma gondii infections in immunocompromised patients, such as HIV-positive individuals[2]. Pyrimethamine alone or in combination with other drugs is reported to be estimated by Liquid chromatography[3-5], Gas –Liquid chromatography[6], liquid chromatography–mass spectrometry[7,8] and HPTLC method[9].
Chemically,Sulphadoxine (SULPH) is 4-Amino-N-(5,6-dimethoxy-4-pyrimidinyl)benzene sulfonamide. It is an ultra-long-lasting sulfonamide often used in combination with pyrimethamine to treat or prevent malaria. It is also used, usually in combination with other drugs, to treat or prevent various infections in livestock[10].Sulphadoxine alone or in combination with other drugs is reported to be estimated by Supercritical fluid chromatography[11], Spectrophotoscopy[12], Microbiological method[13] and Gas chromatography method[14].
Spectrophotometric method is reported for simultaneous estimation of pyrimethamine and sulphadoxine in combination in visible region by making complex. Therefore, the aim of this study is the development of simple, precise, rapid and accurate UV Spectrophotometric Methods for Simultaneous Estimationof Pyrimethamine and Sulphadoxine in Bulk Drug and Pharmaceutical formulations as per ICH guidelines which is easily acceptable as a routine in quality testing laboratories in industry and academic institutes[15].
MATERIALS & METHODS
Instrument, reagents and chemicals
A Shimadzu UV/Visible spectrophotometer, model No.UV-1800 was employed with spectral band width of 2 nm and wavelength accuracy of ± 0.1 nm, with automatic wavelength correction employing a pair of quartz cells. A Sartorius electronic analytical balance was used for weighing the sample. Pure drug sample of pyrimethamine and sulphadoxine were procured from Shreya Life Sciences Pvt. Ltd. Aurangabad, India. Tablet formulation containing Pyrimethamine (37.5mg) and Sulphadoxine (750 mg) was procured from local market. Methanol was used as solvent.
Preparation of stock solution
Standard stock solutions having 100μg/ml of Pyrimethamine and Sulphadoxine were prepared by dissolving separately 10 mg of each drug in 100 ml of methanol. The stock solutions were individually diluted to get final concentration of 10μg/ml each and the diluted solutions were scanned in 200-300 nm range to determine the maximum absorbance (λmax).
Simultaneous equation method (Method I):
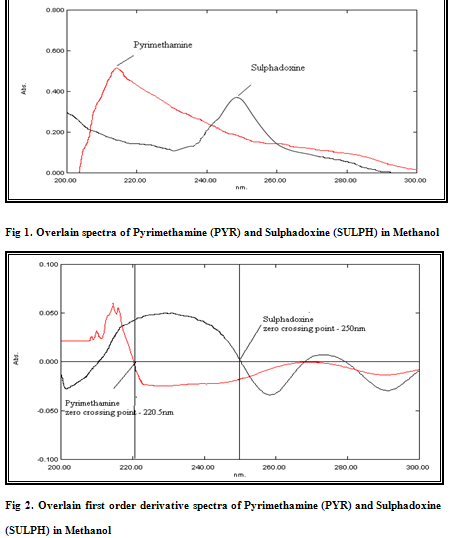
From the stock solution of 100 μg/mL, working standard solutions of drugs were prepared by appropriate dilution and were scanned in entire UV range to determine the max. Pyrimethamine and Sulphadoxine have λmax at 215 nm and 254 nm; respectively [Figure 1].Standard solutions were prepared having concentration 5-30 μg/mL for both drugs. The absorbances of these standard solutions were measured at 215 nm and 254 nm, respectively to plot a calibration curve of absorbance versus concentration. The calibration curves were found to be linear in the concentration range under study. Absorptivities values of Pyrimethamine and Sulphadoxine were determined at selected wavelengths and are presented in Table-1.
Two simultaneous equations (in two variables C1 and C2) were formed using these Absorptivity coefficient values.
A1 = ax1CPYR + ax2CSULPH ………(1)
A1 = ay1CPYR + ay2CSULPH ……….(2)
Where, A1 and A2 are the absorbance of the mixture at 215 nm and 254 nm respectively. ax1and ay1are absorptivities of PYR at 215 nm and 254nm respectively. ax2and ay2 are absorptivities of SULPH at 215 nm and 254 nm respectively. CPYR is the concentration of PYR and CSULPH is the concentration of SULPH in μg/ml.
By applying the cramer’s rule to equation 1 and 2, the concentration CPYR and CSULPH can be obtained as follows.
ax1A2 - ax2A1
CSULPH = ---------------------- ………..(3)
ax1 ay2 - ax2 ay1
And,
ay2A1 - ay1A2
CPYR = ---------------------- ………..(4)
ax1 ay2 - ax2 ay1
[adsense:468x15:2204050025]
First derivative spectroscopy method (Method II):
Standard stock solutions having 100μg/ml of Pyrimethamine and Sulphadoxine were prepared by dissolving separately 10 mg of each drug in 100 ml of methanol. The stock solutions were individually diluted to get final concentration of 10μg/ml each and the diluted solutions were scanned in 200-300 nm range. Data was recorded at the internal of 2nm.Then the spectra of the two drugs were derivatised to obtain first order derivative spectra. After examining the overlain first order derivative spectra, wavelengths were selected where one drug showed zero crossing and other drug showed substantial absorbance. The wavelength selected for Pyrimethamine analysis was 250 nm where Sulphadoxine has zero absorbance and the wavelength selected for Sulphadoxine analysis was 220.5 nm where the absorbance of Pyrimethamine is zero. The derivative spectra of the drugs are shown in Figure 2. Standard solutions were prepared having concentration 5-30 μg/mL for both drugs. The absorbances of these standard solutions were measured at 250 nm and 220.5 nm, respectively to plot a calibration curve of absorbance versus concentration. The calibration curves were found to be linear in the concentration range under study.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Assay of tablet formulation by Method I & II:
20 tablets were weighed and crushed to obtain fine powder. An accurately weighed equivalent weight of tablet powder equivalent to about 10 mg of pyrimethamine and 200mg of sulphadoxine was transferred to 100 mL volumetric flask, dissolved in 20 mL methanol and sonicated for 35 min. The volume was then made up to the mark using same solvent. The resulting solution was filtered through Whatmann filter paper grade I and filtrate was appropriately diluted to get approximate concentration of 1 µg/mL of pyrimethamine and 20µg/mL of sulphadoxine.19μg/mL of pyrimethamine was added externally to obtain the concentration of 20μg/mL of both the drugs in final sample solutions. Absorbances of sample solutions were recorded at 215nm and 254nm and the concentration of two drugs in the sample were determined by using eqns. 3 and 4 (Method-I).
The same tablet sample solutions were subjected to analysis by first order derivative spectroscopy method. The solution was scanned over the wavelength range of 200- 300 nm. 250 nm was used as λmax of pyrimethamine and 220.5 nm as λmax of sulphadoxine. The concentration of each drug was determined by analysis of spectral data of the sample solution with reference to the mixed standards (Method -II).
The analysis procedure was repeated 6 times with tablet formulations. The result of analysis of tablet formulation is reported in Table- 2.
Recovery studies:
To study the accuracy of the proposed methods, recovery studies were carried out by standard addition method at three different levels. A known amount of drug was added to preanalyzed tablet powder and percentage recoveries were calculated. The results of recovery studies were satisfactory and are presented in Table-3.
RESULTS AND DISCUSSION
For all the two methods linearity was observed in the concentration range of 5-30 μg/ml for both pyrimethamine and sulphadoxine. Marketed brand of tablet was analyzed and amount of drug determined by proposed methods ranges from 99.83 to 100.12 as shown in Table 2. The proposed methods were validated as per ICH guideline. The accuracy of method was determined by calculating mean percentage recovery. It was determined at 80,100 and 120 % level. The % recovery ranges from 99.60% to 100.29% for all the three methods and are presented in Table 3.Precision was calculated as repeatability (% RSD is less than 2) and inter and intraday variations (%RSD is less than 2) for both drugs. The repeatability data, ruggedness data are presented in Table-3.
TABLE – 1: ABSORPTIVITY VALUES (E 1%, 1 CM) OF PYRIMETHAMINE (PYR) AND SULPHADOXINE (SULPH) AT 215 NM AND 254 NM (METHOD -I)
|
Sr.No. |
Absorptivity at 215nm |
Absorptivity at 254nm |
||
|
|
PYR |
SULPH |
PYR |
SULPH |
|
1 |
472.00 |
190.00 |
172.00 |
392.00 |
|
2 |
471.00 |
187.00 |
171.00 |
389.00 |
|
3 |
473.33 |
188.00 |
170.67 |
388.67 |
|
4 |
474.50 |
189.00 |
170.50 |
394.50 |
|
5 |
472.40 |
189.60 |
171.20 |
390.80 |
|
6 |
473.67 |
189.00 |
173 |
392.67 |
|
Mean |
472.82 |
188.77 |
173.39 |
391.27 |
|
±S.D. |
±1.1520 |
±1.0027 |
±0.8629 |
±2.0439 |
|
RSD% |
0.2464 |
0.5311 |
0.5034 |
0.5223 |
TABLE-2: RESULTS OF SIMULTANEOUS ESTIMATION OF MARKETED FORMULATION FOR METHOD I AND II
|
Method |
Tablet content |
Label claim (mg/Tablet) |
Label claim (%) |
±S.D. |
RSD (%) |
|
|
|
|
|
|
|
|
Method- I |
PYR |
37.5 |
100.02 |
±0.1557 |
0.1556 |
|
SULPH |
750 |
99.86 |
±0.3133 |
0.3137 |
|
|
Method- II |
PYR |
37.5 |
100.12 |
±0.2164 |
0.2161 |
|
SULPH |
750 |
100 .02 |
±0.4228 |
0.4226 |
TABLE-3: RESULTS FOR RECOVERY STUDIES
|
Level of recovery |
Amt of drug added µg/ml |
Drug |
Method I |
Method II |
||
|
Recovery (%) |
±SD |
Recovery (%) |
±SD |
|||
|
80% |
16 |
PYR |
100.28 |
±0.020 |
100.02 |
±0.038 |
|
16 |
SULPH |
99.60 |
±0.055 |
100.15 |
±0.122 |
|
|
100% |
20 |
PYR |
100.23 |
±0.081 |
99.91 |
±0.072 |
|
20 |
SULPH |
99.72 |
±0.125 |
100.29 |
±0.047 |
|
|
120% |
24 |
PYR |
100.20 |
±0.065 |
99.97 |
±0.075 |
|
24 |
SULPH |
99.74 |
±0.102 |
99.96 |
±0.284 |
|
CONCLUSION
The proposed methods were found to be simple, accurate and rapid for the routine determination of pyrimethamine and sulphadoxine in tablet formulation. To study the validity and reproducibility of proposed methods, recovery studies were carried out. The methods were validated in terms of linearity, accuracy, precision, specificity and reproducibility. The two methods can be successfully used for simultaneous estimation of pyrimethamine and sulphadoxine in combined dosage form.
REFERENCES
1. O’Neil MJ. The Merck Index: an Encyclopedia of Chemicals, Drugs and Biologicals.14th ed. Whitehouse Station: Merck Research Laboratories, Division of Merck & Co., Inc., 2006. p 1125.
2. Perfitt K.Martindale: The Complete Drug Reference, 35th ed. London: Pharmaceutical Press; 2007.p 1546.
3. Minzi O, Massele A, Gustafsson L, Ericsson O. Simple and cost-effective liquid chromatographic method for determination of pyrimethamine in whole blood samples dried on filter paper. J. Chromatogr. B 2005; 814: 179–83.
4. Na-Bangchang K, Tanartya R, Ubalee R, Kamantkom B, Karbwang J. Alternative method for determination of pyrimethamine in plasma by high-performance liquid chromatography. J. Chromatogr. B 1997; 689: 433-37.
5. Edstein M. Simultaneous measurement of sulphadoxine, N.,-acetylsulphadoxine and pyrimethamine in human plasma. J.Chromatogr 1984; 306:502-07.
6. Jones CR, Ryle PR, Weatherly BC. Measurement of pyrimethamine in human plasma by gas-liquid chromatography. J. Chromatogr 1981; 224: 492-5.
7. Koesukwiwat U, Jayanta S, Leepipatpiboon N. Validation of a liquid chromatography–mass spectrometry multi-residue method for the simultaneous determination of sulfonamides, tetracyclines, and pyrimethamine in milk. J. Chromatogr. A 2007; 1140:147–56.
8. Sabarinath S, Singh RP, Gupta RC. Simultaneous quantification of α/β diastereomers of arteether, sulphadoxine and pyrimethamine: A promising anti-relapse antimalarial therapeutic combination by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2006; 842: 36–42.
9. Shewiyo DH. Development and validation of a normal-phase high-performance thin layer chromatographic method for the analysis of in pyrimethamine and sulphadoxine in sulfamethopyrazine tablets. J. Chromatogr. A 2009; 1216: 7102–07.
10. The elements of Pharmacology by Derasari and Gandhi. B.S.Shah prakashan, 18th ed, 2009. p 556.
11. Bhoir SI, Bhoir IC, Bhagwat AM, Sundaresan M. Determination of sulphadoxine in human blood plasma using packed-column supercritical fluid chromatography. J. Chromatogr. B 2001; 757:39 –47.
12. Onah JO, Odeiani JE. Simultaneous spectrophotometric determination of sulphadoxine and pyrimethamine in pharmaceutical formulations. J. Pharm. Biomed. Anal 2002; 30: 851–7.
13. Weidekamm E, Plozza-Nottlebrock H, Forgo I, Dubach U. A microbiological method for quantifying Sulphadoxine and Pyrimethamine. WHO 1982; 60: 115.
14. Bonini M, Mokofio F, Barazi S. Gas Chromatographic method for the determination of sulphadoxine and pyrimethamine in blood and urine. J. Chromatogr 1981; 224:332.
15. ICH, Q2 (R1): Validation of analytical procedures methodology: Text and Methodology, International Conference on Harmonization, Geneva, November 2005.ich.org/LOB/media/MEDIA417.pdf.[accessed on 2010 dec 14].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









