About Author
Nrusingha Panda
Department of Quality Assurance,
Mankind Pharma Ltd., Bermiok Elaka,
South Sikkim, Sikkim, Zip Code- 737126, India
ABSTRACT
Regulatory authorities expect from pharmaceutical industries that there should be written and approved procedures of sampling to collect samples for testing before approval of components of drug products, container and closure of drug products and drug product itself to assure patient safety in all respects whether chemical, physical and biological point of view of the drug product. In Pharmaceutical industries manufacturing oral solid dosage formulations, there are many critical steps which are performed by manually or automatically or by combination of both. Among those critical steps, one step is performing sampling of different materials such as starting materials, packaging materials, in-process materials, finished product. The review focused on sampling procedure used for the above mentioned materials and sampling tools used for performing sampling activities in pharmaceutical industries.
INTRODUCTION
In Pharmaceutical industry, sampling process plays a major role. Sampling is having regulatory requirements and also from business point of view, a Pharma industry has to test a lot or a batch in the testing laboratories before getting release the lot or batch in to the market. Several problems arise in the Pharma industries many a times due to wrong techniques used during sampling. So Pharma industries apply much stress in preparing a Standard operating procedure (SOP) or protocol and training of concerned persons on sampling.
SAMPLING
Sampling is a process by which one can prepare a sample out of one lot or a batch. Sampling may have various reasons such as but not limited to acceptance of a lot or batch, acceptance of consignment, pre-qualification, in-process control, batch release testing, retention or control sample with drawl, verification of identity, adulteration. Sampling plays an important part of a system of Quality Assurance.
SAMPLE
Sample is a portion of a material collected according to a defined sampling procedure. Sample represents a whole batch or a lot. Sample is generally prepared for analysis purpose to evaluate quality of a drug substance or drug product. A lot or batch can be accepted or rejected based on the pass or fail results of respective sample.
Samples shall be representative and adequately identified. Procedures shall be established such as appropriate retesting procedure including frequency of retesting of any component, drug product container, or closure that is subject to deterioration.
REQUIREMENTS OF SAMPLING
• Written and approved SOP on sampling including authorized persons for sampling.
• Availability of sampling facilities designed in a way to prevent contamination and cross contamination (e.g. sampling booth in case of raw materials sampling and individual production area or line for in-process sampling).
• Sampling equipment or instrument or accessories (e.g. sample containers, sampling thieves and bullets or dies, weighing balances, butter paper, spatula etc.)
• Sample plan which describes stages of sampling, nos. of locations of sampling, quantity of sample from each location, stage of sampling (e.g. in tabular form describing the sampling plan).
• Protocol describing sampling method and sampling plan and report to record the sampling details.
• Trained personnel who have adequate training on different disciplines with respect to correct sampling such as: training on sampling plan, sampling procedure, SOP, protocol, techniques and equipments to be used for sampling, prevention of contamination and cross contamination, visual observation on appearance of materials during sampling, verification of containers and their labels affixed on them, importance of highlighting and recording observations regarding any unexpected and unusual circumstances.
PRECAUTIONS TO BE TAKEN FOR SAMPLING
Following precautions shall be taken:
• Proper labelling of sample indicating material/ item/ product name, A.R. no./ batch no./ lot no., manufacturing date, expiry date, location no., stage of sampling, quantity of sample, date of sampling, signature of sampler etc.
• Storage condition of sample shall be maintained as appropriate.
• Sampling tools (e.g. sampling thief, die, spatula etc.) used for sampling of raw materials or granules or blend should be made up of Stainless steel i.e. SS 316 or SS 316L or any suitable inert material since these tools shall be in direct contact with the materials or products from where sample is to be collected.
• Container for collection and storage of sample shall be made of some inert material which will not interact with the sample stored.
• Sampling shall be done in a manner to prevent contamination. To prevent contamination, cleaned and dried containers shall be used after thorough verification for the cleanliness and dryness of the containers, closure of the container shall be sealed immediately after putting the collected sample into the container.
• For light sensitive materials, amber coloured glass bottles, black colour self sealing polybag, containers wrapped in aluminium foil shall be used.
• Verification of cleanliness and dryness of sampling tools such as sampling thief, die etc. is important to prevent contamination and cross contamination.
TYPES OF SAMPLING METHODS USED
Random Sampling
• In this method of sampling, any container in a consignment or any portion of a container can be chosen by chance.
• For example, in case of sampling only 10 nos. of containers out of 100 nos. of containers, any 10 nos. of containers can be selected randomly for sampling purpose. Out of the 10 nos. of containers, sampling can be done randomly from top, middle and bottom portion of any container.
Systematic Sampling
• In this method of sampling, at a particular and regular interval sampling can be done. The interval may be time or a number.
• For example, if 10 nos. of containers are to be sampled out of 100 nos. of containers, then each container can be selected at every 10th no. of container, e.g. 10th, 20th, 30th, 40th... so on nos. of containers.
• Another example where a particular time interval can be chosen as a method of systematic sampling is that sampling can be done at a regular time interval such as 30 minutes, 60 minutes or 120 minutes to collect composite sample of a particular batch during its processing.
Stratified Sampling
• Stratified sampling is the process of sampling dosage units at predefined intervals and collecting representative samples from specifically targeted locations in the compression/filling operation that have the greatest potential to yield extreme highs and lows in test results.
• The term “Stratified” means dividing in to sub groups (Strata). Strata should be identified as worst locations from which if sample is collected have the greatest chance of failure. If the samples collected from the strata pass the predefined acceptance criteria, then we can say that the whole batch passes the acceptance criteria. Generally the stratified sampling is done during Process performance qualification (PPQ) or validation of products.
• Regular interval used in case of systematic sampling is slightly different than that used in case of stratified sampling in a way that in systematic sampling is done for regular batches and stratified sampling is done in PPQ or validation batches and one composite sample is prepared in case of systematic sampling while in stratified sampling individual samples taken at regular intervals are analysed separately.
• Example of Stratified sampling: Collecting 7 dosage units from at least 20 locations or collecting 3 dosage units from at least 40 locations during compression or capsule filling operation.
Pooled Sampling
• Pooled sampling means pooling or collecting a sample out of a collected sample.
• For example: Suppose for conducting analysis of a product, 100 nos. of dosage units are required after compression of a batch. And in a systematic sampling i.e. collecting samples either at regular time interval or at particular container no., suppose a sample of 1000 nos. of dosage units is collected. Out of the sample of 1000 nos. of dosage units, random sampling of 100 no. units is done for laboratory analysis purpose. So a composite sample of 100 nos. of dosage units is collected out of a composite sample of 1000 nos. of dosage units which is an example of pooled sampling.
SAMPLING ERRORS
Sampling error can happen in any stage of sampling such as in case of starting materials, powder blend, in-process material, finished product.
There may be different types of sampling errors as mentioned below but not limited to:
Human error
• Any error that occurs due to involvement of human during sampling which may lead to unexpected result is known as human error. Human error may lead to a deviation or incidence.
• For Example: Spillage of sample outside the container of the sample, collecting sample in wrong container or container labelled with another product label, selection of wrong sampling tools or containers, collection of wrong quantity of sample, inadequate storage condition of the collected sample, improper handling of sampling tools.
• Human error may occur due to ignorance, if sampler is not trained enough on a particular SOP or protocol, not following procedure mentioned in the SOP or protocol.
Sampling tool error
• Wrong design of sampling tools such as sampling thief or die, reaction of the material of construction (MOC) of sampling tool with the product during sample collection, improper filling of powder blend in to the die cavity can lead to errors in sampling.
• For example: Leaking of powders from the die cavity after closing of the sampling thief just after sample collection may lead to variation in sample mass which may in turn lead to variation in result.
Unknown error
• Mixing up of different samples with each other or contamination of sample during sampling or storage, if powder bed disturbed or segregated during sampling due to unknown reasons lead to variation in results or unexpected results.
• For example: Sample containers verified visually as clean but somehow contaminated the sample put into it and/ or in some cases due to improper storage conditions if moisture develops in the container may lead to false results.
PROCEDURE FOLLOWED IN DIFFERENT STAGES OF SAMPLING
Different Sampling stages in Solid Oral dosage (SOD) formulation are as mentioned below:
RAW MATERIAL SAMPLING
Separate areas may be employed in sampling of Active Pharmaceutical Ingredients (API) and excipients. Or else, one area can be used for sampling of both by doing cleaning of sampling booth and equipment [e.g. Reverse Laminar Airflow (RLAF)].
Sampling procedure:
• Establish sample quantity needed to be sampled for each raw material. The sample quantity must be sufficient enough to carry out one full analysis plus some extra quantity in case of any out of specification (OOS)/ out of trend (OOT) investigation and the sample quantity should also include the retention/ control sample quantity.
• Identify the consignment by respective label and Goods receipt note (GRN). Take all the containers to the sampling area one by one.
• Open the container under RLAF and sample the required quantity using a clean sampling thief from top, middle and bottom portions of the container. Make a composite sample by using samples taken from all the containers. Label the container containing the composite sample.
• In case of API, sample from all the containers of a consignment shall be withdrawn i.e. 100% sampling shall be done. In case of excipients, by using the formula n = 1 + √N, sampling can be done where, n= nos. of containers from which sample to be withdrawn, N= Total nos. of containers in the consignment. For example, in case of sampling of excipients, if there are 100 nos. of containers in a consignment, then using the above formula n= 1 + √100 = 1+10= 11 nos. of containers shall be taken for sampling purpose. If value of ‘N’ is less than or equal to 4, then all the containers shall be taken for sampling purpose. The value of ‘n’ should be rounded to the next integer if ‘n’ value comes as a decimal digit.
• There is some regulatory concern using the above mentioned formula. According to regulatory authorities, it is improbable that a reduced sampling and testing would be accepted for:
a) Starting materials supplied by intermediaries such as brokers where the source of manufacture is unknown or not audited.
b) Starting materials for use in other than SOD products especially in parenteral products.
• There should be a validated procedure in accordance with the regulatory guidelines that would permit less than all containers to be sampled and tested.
PACKAGING MATERIAL SAMPLING
Packaging materials shall be sampled in a way as to represent a consignment, and examined or tested after receipt of packaging materials. Care should be taken by the sampler to prevent mix up of packaging materials during sampling. As a precaution, samples of packaging materials should never be returned to the consignment to avoid mix up.
Sampling procedure
• Establish sample quantity needed to be sampled for each type of material i.e. primary and secondary packaging materials. The sample quantity must be sufficient enough to carry out one full analysis plus some extra quantity in case of any out of specification (OOS)/ out of trend (OOT) investigation and the sample quantity should also include the retention/ control sample quantity.
• Identify the consignment by respective label and Goods receipt note (GRN).
• Primary packaging materials shall be sampled under laminar air flow (LAF) to prevent environmental contamination.
• In case of primary packaging material, 100% sampling shall be carried out except bottles, polypropylene caps. For bottles and polypropylene caps, formula n = 1 + √N can be followed as mentioned in case of sampling of raw materials.
• In case of secondary and tertiary packaging materials, random sampling shall be done by opening different containers / Boxes as per formula n = 1 + √N.
IN-PROCESS MATERIAL SAMPLING
Blending Stage
• For routine batches, generally sample is collected after unloading of granules in to containers.
• According to the sampling plan, sample shall be collected from all the containers containing blend. Sample from each container shall be collected from top, middle and bottom portions of the container by using a sampling thief.
• A composite sample shall be prepared by taking all the collected samples in to one container such as a fresh and virgin self sealing polybag.
Compressed Tablet
• After completion of compression of a batch, sample shall be collected from all the containers randomly in a fresh and virgin self sealing polybag to make a composite sample as per sampling plan.
• Alternatively, another sampling procedure can be adopted. Sampling frequency and nos. of samples required at each sampling interval can be decided based on the batch size of a product and compression machine speed (i.e. total batch run time) and the required quantity of sample as per sampling plan. As per the sampling frequency, samples can be collected in a container or fresh and virgin self sealing polybag to make a composite sample. The sampling frequency must include start and end of the total batch run time.
Coated Tablet
• Sampling of coated tablets can be performed after completion of the coating process directly from the coating pan or from the containers after unloading of the coated tablets.
• Collect the sample as per sampling plan in a fresh and virgin self sealing polybag to make a composite sample.
Filled Hard gelatine capsule
• After completion of capsule filling of a batch, sample shall be collected from all the containers randomly in a fresh and virgin self sealing polybag to make a composite sample as per sampling plan.
• Alternatively, another sampling procedure can be adopted. Sampling frequency and nos. of samples required at each sampling interval can be decided based on the batch size of a product and capsule filling machine speed (i.e. total batch run time) and the required quantity of sample as per sampling plan. As per the sampling frequency, samples can be collected in a container or fresh and virgin self sealing polybag to make a composite sample. The sampling frequency must include start and end of the total batch run time.
FINISHED PRODUCT SAMPLING
• Finished product sampling shall be done at secondary packing at start, middle (at a defined frequency) and end of the packing operation.
• Sample shall be collected as per sampling plan. The sampling plan may include samples such as but not limited to sample for laboratory analysis, microbiological analysis sample, Retention/ control sample, Stability sample.
PROCESS VALIDATION SAMPLING
Blending Stage
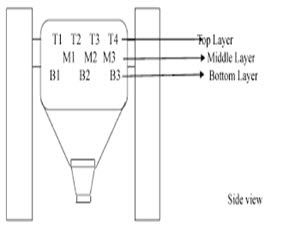
• Sampling shall be performed from the blending machine in which the product is blended. As per sampling plan, sampling shall be performed from top, middle and bottom portions of the blender using a sampling thief and required size of dies from different locations depending on the design of the blender and regulatory requirements. Sample size may vary from 1X to 3X of the average weight of one unit dose. The size of required dies can be determined by using bulk density of the blend.
• Samples collected from different locations shall be put in to different containers with proper labelling.
• Composite sample can also be prepared by taking samples from top, middle and bottom portions of the blender or by taking samples from all the containers after unloading of the blend.
• One can determine the top, middle and bottom portion of a blender as a depth of approximately 25%, 50% and 75% respectively from the top layer of the blend.
• During sampling, a distance of about 0.5 feet shall be kept for the sampling thief from the wall of the blender.
Compressed Tablet
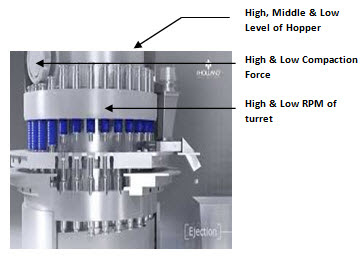
• Sampling can be performed directly from the compression machine from one side of the machine (for single rotary compression machine) and from both the sides of the compression machine (for double rotary machine).
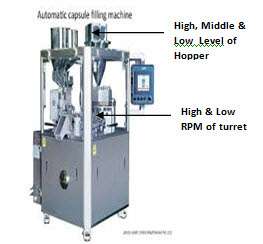
• Samples shall be withdrawn for various challenge tests such as but not limited to variation in compaction force of the machine, variation in speed of the machine, from high, middle and low level of powder in the hopper of the compression machine.
• Sample shall be withdrawn for optimum parameters of the compression machine as per procedure mentioned in point C for compressed tablet.
Coated Tablet
• Same procedure shall be followed as mentioned in point C for coated tablet.
Filled Hard gelatine Capsule
• Sampling can be performed directly from the capsule filling machine for various challenge tests such as but not limited to variation in speed of the capsule filling machine, from high, middle and low level of powder in the hopper of the capsule filling machine.
• Sample shall be withdrawn for optimum parameters of the capsule filling machine as per procedure mentioned in point C for filled hard gelatine capsule.
Finished Product
• Sampling can be performed directly from the blister packing machine or strip packing machine in the primary packing area.
• Sampling shall be done for various challenge tests such as but not limited to variation sealing temperature, variation in speed of the packing machine.
• Under normal operating parameters, sampling shall be performed as mentioned in point no. C of finished product sampling.
ACCEPTANCE QUALITY LEVEL (AQL) SAMPLING
• There are various sampling plans such as single sampling plan or multiple sampling plans for performing AQL.
• AQL sampling is performed to identify and accept nos. of defective units (i.e. critical, major and minor defects) in a lot or a batch.
• AQL sampling can be performed at stages such as but not limited to after compression, during online compression, after coating, after filling of capsules, during online filling of capsules.
• Samples shall be withdrawn as per AQL chart.
• During online processing of a batch, sample size at a particular frequency can be calculated as per below mentioned formula:
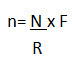
Where, n= sample size at each interval
N= Total no. of samples
R= Total run time of machine in minutes
F= Frequency or time interval in minutes
• Sampling can also be performed after processing of a batch by dividing the total sample size with the total nos. of containers containing the batch. Sampling shall be performed by taking equal nos. of samples from individual containers randomly.
TYPES OF SAMPLING TOOLS USED IN PHARMACEUTICAL INDUSTRY IN SOD FORMULATION
A) Scoops

B) Sampling thief

C) Spatula

D) Bullets or Dies

SAMPLING LOCATIONS IN DIFFERENT EQUIPMENTS
Blender
Contabin blender or Octagonal blender

Compression
Coating

In-process container
Capsule filling machine
Blister packing machine

Strip packing machine

CONCLUSION
Sampling performed in various stages of solid oral dosage formulation manufacturing industries plays a critical role in lot or batch acceptance, investigation in case of any deviation or incidence which in turn helps to maintain or improve or develop the quality of a product according to regulatory or in house requirements of a pharmaceutical industry in view of patient safety.
Sampling methods other than those mentioned above can be adopted having sound scientific justification and rationale. There may be many more sampling errors associated with sampling other than those mentioned in the article. Sampling errors should be identified and can be avoided by regular training and monitoring of samplers and/or by performing sampler validation in sampling activities.
REFERENCES:
1. WHO guideline for sampling of pharmaceutical products and related materials, WHO TRS No. 929, Annex 4, 2005.
2. Sampling of starting and packaging materials, Eudralex, Volume 4, Annex 8, 2011.
3. Current good manufacturing practices for finished pharmaceuticals, 21 CFR, part 211, 2019.
4. Good manufacturing practices and requirement of premises, plant and equipment for pharmaceutical products, Schedule M, 2001.
5. Sampling of starting and packaging materials, Eudralex, Volume 4, Annex 8, PICS, 2018.
6. Sampling of starting and packaging materials, Eudralex, Volume 4, Annex 8, TGA, 2019.
7. Guidance for industry, Powder blends and Finished dosage units- Stratified In-process dosage unit sampling and assessment, Draft guidance, USFDA, 2003.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









