 About Author: Mr. Mahesh W. Thube*, Dr. Sadhana R.Shahi, Mr. Abhay Padalkar
About Author: Mr. Mahesh W. Thube*, Dr. Sadhana R.Shahi, Mr. Abhay Padalkar
Mr. Mahesh W. Thube*: Department of Pharmaceutics,
Government College of Pharmacy, Aurangabad - 431 005, Maharashtra, India
Dr. Sadhana R. Shahi: Assisstant Professor, Govt. College of Pharmacy, Aurangabad, Department of Pharmaceutics.
Abstract
Ion exchange resin (IER) is high molecular weight polyelectrolyte having charged functional site. IER are chemically vinyl, divinyl benzene and polystyrene copolymers. IER in past years have received extensive attention by pharmaceutical industry due to their versatile application. Previously IER were mainly used for water purification only but recently they have been studied for Novel Drug Delivery System. IER are mainly used for taste masking but, they also possess modifying release properties. The IER are complexed with drug to form resinates by batch process or column process. If necessary the resinates are coated with polymeric material by microencapsulation technique. Coated resinates acts as a controllable rate limiting factor for exchange of ions and also for exchange of drug, thus, modifying the release of drugs. The review article highlights the application of sustained and controlled release resinate for the development of various drug delivery system.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1113
Introduction1
The modern drug delivery system is capable of producing not only sustained release, but also controlled release (i.e., a release rate that is not greatly in?uenced by the gastrointestinal environment) drug delivery system. The oral controlled-release system is usually made of polymers, and the mechanisms of release are generally regulated by diffusion, bioerosion or degradation, swelling or generation of osmotic pressure or exchange of ions. While signi?cant advances have been made in the development of elegant systems to modify the oral delivery of drugs, the basic approaches has remained largely unchanged with the major systems being:
1. Insoluble, slowly eroding, or swelling matrices,
2. Polymer-coated tablets, pellets, or granules,
3. Osmotically driven systems,
4. Systems controlled by ion exchange mechanisms, and
5. Various combinations of these approaches.
Sustained release indicates an initial release of drug sufficient to provide a therapeutic dose soon after administration, and then a gradual release over an extended period. Sustained releases are dosage form that provides medication over an extended time. Controlled release system is able to provide some actual therapeutic control, whether it is of a temporal nature, spatial nature or both. Thus, the system attempts to control drug concentration in the target tissue. This correctly suggests that there are sustained release systems that cannot be considered controlled release.
The important drawback of conventional controlled or sustained release system is dose dumping but the use of IER has solved this problem. The polymeric (physical) and ionic (chemical) properties of IER will release the drug more uniformly than that of simple matrices (because of physical properties only). The better drug-retaining properties and prevention of dose dumping of IER place them as a suitable candidate for sustained or controlled release system.
The review enlightens the emerging application of Ion exchange resin for sustained/controlled release drug delivery system. It also describes the mechanism of ion exchange process, various systems of resin and marketed available resin with their application.
1.1 Advantages and limitations of a drug formulated into an extended release (ER Dosage form2:
1.1.1 Clinical advantages
1. Reduction in frequency of drug administration
2. Improved patient compliance
3. Reduction in drug level fluctuation in blood
4. Reduction in total drug usage when compared with conventional therapy
5. Reduction in drug accumulation with chronic therapy
6. Reduction in drug toxicity (local/systemic)
7. Stabilization of medical condition (because of more uniform drug levels)
8. Improvement in bioavailability of some drugs because of spatial control
9. Economical to the health care providers and the patient
1.1.2 Commercial/industrial advantages
1. Illustration of innovative/technological leadership
2. Product life-cycle extension
3. Product differentiation
4. Market expansion
5. Patent extension
1.1.3 Potential limitations
1. Delay in onset of drug action
2. Possibility of dose dumping in the case of a poor formulation strategy
3. Increased potential for first pass metabolism
4. Greater dependence on GI residence time of dosage form
5. Possibility of less accurate dose adjustment in some cases
6. Cost per unit dose is higher when compared with conventional doses
7. Not all drugs are suitable for formulating into ER dosage form
1.2 Types of Oral Sustained or Controlled release system3 :
1. Dissolution Controlled Systems
2. Diffusion Controlled systems
a) Reservoir System
b) Matrix System
3. Bio erodible Matrix System and Combination of Dissolution and Diffusion Controlled system:
4. Osmotic Systems
5. Ion Exchange Systems
1.2.1 Dissolution Controlled Systems:
As the name indicates the release of drug will be limited by the rate of dissolution.
The two main types are:
a) Encapsulated dissolution Product: The approach is achieved by preparing appropriate salt or derivatives, coating the drug with a slowly dissolving material.
b) Matrix Dissolution Product: Incorporating it into tablet with a slowly dissolving carrier.
1.2.2 Diffusion Controlled systems:
These are characterized by release rate of a drug being dependent on its diffusion through an inert membrane barrier. Two main subclasses are:
a) Reservoir System:
1. The core of drug, the reservoir, surrounding by a polymeric membrane.
2. The nature of membrane determines the rate of release of drug from reservoir.
b) Matrix System:
Homogeneous dispersion of solid drug in polymer mix
[adsense:468x15:2204050025]
1.2.3 Bio erodible Matrix System and Combination of Dissolution and Diffusion Controlled system:
It is the homogeneous dispersion of drug in an erodible matrix and release rate varies with different polymer. It’s difficult to control kinetics owing to multiple processes of release.
1.2.4 Osmotic Systems:
In this system Osmotic pressure provides the driving force to generate the controlled release of drug. The drug is surrounded by semi permeable membrane and release is governed by osmotic pressure.
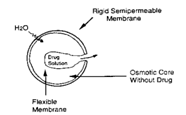
Fig I: working of Osmotic system
2 IER (Ion Exchange Resin)4:
Ion exchange resins (IER) may be defined as high molecular weight water insoluble polymers containing fixed positively or negatively charged functional groups in their matrix, which have an affinity for oppositely charged counter ions. IER are solid insoluble high molecular weight poly electrolytes that can exchange with surrounding medium reversibly and Stochiometrically.
IER are Styrene (Di Vinyl Benzene) copolymer containing
- Acidic groups: Carboxylic or sulphonic for Cation E.R.
- Basic groups: Quaternary Ammonium for Anion E.R
Based on the nature of the ionic species being interchanged, the IE process is known as either cation exchange (CE) or anion exchange (AE).The IE process is competitive in nature. In practice, drug in an ionic form (usually solution) is mixed with the appropriate IER form a complex, known as ‘resinate’.
The Performance of Resinate Is Governed By Several Factors, Such As5:
1. The pH and temperature of the drug solution;
2. The molecular weight and charge intensity of the drug and IER;
3. Geometry;
4. Mixing speed;
5. Ionic strength of the drug solution;
6. Degree of cross linking and particle size of the IER;
7. The nature of solvent; and
8. Contact time between the drug species and the IER.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2.1 Mechanism of Ion Exchange Process6 :
The interactions between the IER and drug, although primarily chemical in nature, are also partially a result of physical adsorption. These interactions are commonly referred to as ‘adsorption on IER’, rather than Complexation on most occasions. The IE process, therefore, is generally regarded as a double-decomposition process, in which the IER used is able to provide the type of ion required to replace the one that is adsorbed from the solution.
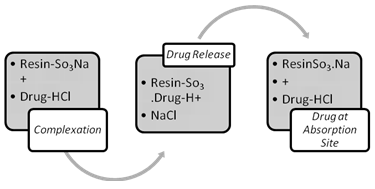
Fig II: Complexation and Release of Drug from IER
2.2 Physical Properties, Chemistry and Classification of IER6:
In general, IER consist of spherical beads of approximately 0.5–1.2 mm in diameter. The most common type is an opaque yellow in color, although other colors are also reported. The constitution of each spherical particle of IER is similar to that of a homogeneous gel. The shrinkage or expansion of the spherical volume that takes place is based on the ionic environment in which the IER is present.
Table I: Functional Group in IER
|
Functional Group in IER |
Exchange Species |
|
|
Cationic Exchange Resin |
||
|
Strongly acidic |
Sulphonic acid or |
-SO3H |
|
Phosphonic acid |
- SO3P |
|
|
Weakly acidic |
Carboxylic acid or Phenolic |
-COOH |
|
AnionicExchange Resin |
|
|
|
Strongly basic |
Quaternary Ammonium |
N+R3
|
|
Weakly basic |
Primary, secondary or tertiary amine |
H2NR1, HNR2,NR3 |
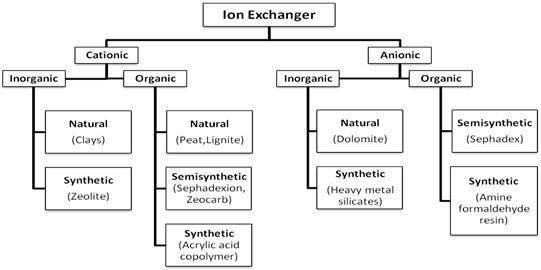
2.3 Classification of IER6:
Classification of IER
2.4 Selection of suitable IER:
The selection of IER mainly based on the following factors
1. Ion exchanging Capacity of the IER i.e. the concentration of the exchangeable group in the resin, usually expressed in meq /g of dry resin.
2. Degree of cross linking in the resin matrix;
3. Particle size of resin;
4. Nature of drug and site of drug delivery.
5. Swelling ratio
6. Biocompatibility and biodegradability; and
7. Regulatory status of the IER
2.5 Characterization of IER7 :
The quality of IER reflects the performance of the IER, so it is important to evaluate the characteristics of IER.
Table II: Various characterization methods of IER
|
Sr.no |
Characteristic |
Method |
|
1. |
Particle Size |
- Screening by Micro sieves, Microscopy, Coulter Counter |
|
2. |
Porosity |
- Nitrogen Adsorption at -195o C, True displacement by Mercury Displacement, Air compression Pychnometer |
|
3. |
Moisture Content |
- Karl Fisher titrimetry |
|
4. |
Ion Exchange Capacity |
Strong Cationic ER- by evaluating the amount of moles of sodiumwhich are absorbed by dry resin in the hydrogen form. |
|
Strong Anionic ER- by evaluating the amount of moles of chlorine which are absorbed by dry resin in the hydroxide form. |
2.6 Controlled or Sustained Release System of IER5:
A major drawback of controlled or sustained release systems is dose dumping, resulting in increased risk of toxicity. Ion exchange resins offers better drug retaining properties and prevention of dose dumping. The polymeric (physical) and ionic (chemical) properties of ion exchange resin will release the drugs more uniformly than that of simple matrices (because of physical properties only).
2.6.1 Resinates:
Drug loaded onto the strong IER resinates provides simplest form of controlled or sustained release delivery system. Resinates can be filled directly in a capsule, suspended in liquids, suspended in matrices or compressed into tablets. Drug will be slowly released by ion exchange phenomenon and absorbed.
2.6.2 Microencapsulated or Coated resinates8:
Microencapsulation of resinates provides better control over the drug release because of presence of rate controlling membrane. The absorption of the drug from coated resinates is a consequence of the entry of the counter ions into the coated resinates and release of drug ions from drug resin complex by the ion exchange process and diffusion of drug ions through the membrane into the dissolution medium. Designed release rate at the desired level can be obtained by optimization of coating thickness. Microencapsulation of resinates can be achieved by air suspension coating (Wurster process), interfacial polymerization, solvent evaporation or pan coating.
2.6.3 Pennkinetic System9,10:
Modification of the coating of resinates for improved monitoring of the drug release pattern has been the concept of Pennkinetic system. In this system, it is pretreated with polyethylene glycol 400 to maintain the geometry and improve coating process. The pretreated resinates are then coated with ethyl cellulose or any other water insoluble polymer, polyethylene glycol helps in controlling the swelling rate of matrix in water, while an outer ethyl cellulose coating modifies the diffusion pattern of ions in and out of system. The 12 hr cough products of Pennwalt Corp. is as follows:
Table III: Pennkinetic system Brands
|
Brand |
Active drug |
|
Delsym |
Dextromethorphan |
|
Corsym |
Chlorpheniramine |
|
Cold Factor 12 |
Phenyl propanolamine |
2.6.4 Hollow Fiber System11:
The system has the advantage of high surface area to volume ratio, loading flexibility, membrane permeability and potentially slower GI transit time. These characteristics provide a method to obtain controlled release for drugs in the small intestine/or in colon. Hollow fibers made from suitable polymeric materials are filled with resinate to obtain a controlled or sustained release profile. Biodegradable hollow fibers can be used for drug delivery in the form of implants.
2.6.5 Pharmazomes1:
Elan Corporation has also developed a liquid sustained-release system called Pharmazome. The company has not disclosed the mechanism of action of the system; it is believed to provide the same bene?ts of long action and taste masking offered by the Pennkinetic system.
2.6.6 Resin and Drug binding factors58:
Table IV: Drug and respective resins binding nature
|
For binding of Basic Drug to Cation Exchange Resin |
For Binding Acidic Drug, to Anion Exchange Resin |
|
(A) Resin (Na-Form) Plus Drug (Salt Form) (B) Resin (Na-Form) Plus Drug (As Free Base) (C) Resin (H-Form) Plus Drug (Salt Form) (D) Resin (H-Form) Plus Drug (As Free Base) |
(A) Resin (Cl-Form) Plus Drug (Salt Form) (B) Resin (Cl-Form) Plus Drug (As Free Acid) (C) Resin (As Free Base) Plus Drug (Salt Form) (D) Resin (As Free Base) Plus Drug (As Free Acid) |
2.6.7 Purification and Activation of Resin:
Resins are prepared before the actual use for two main purposes: to removes impurities and to activate the resin. It is done mainly by cyclic treatment of various chemicals and demonized water.
Examples of various chemicals: 1N NaOH, 1N HCl, 50%/95% Ethanol, Methanol.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2.6.8 Formation of Drug-Resin complex i.e. ‘Resinate’5:
The drug may be complexed with the ion exchange resin by a column or batch process. Since the reaction is an equilibrium phenomenon, maximum potency and efficiency is best obtained by the column process. However, the batch process is simpler and quicker than column process, only alternative to be used for very fine particles.
1. Batch process:
A typical batch procedure involves slurring the resin in water, filtering or decanting the liquid on top, slurring the resin with the desired acid, base, or salt solution to change cycle if necessary, decanting, and washing with water several times, and treating with the appropriate drug solution. After adsorption, the complex formed should be washed with water and dried.
2. Column method:
In a typical column procedure for preparing complex of an amine drug on a strong cation exchange resin, the resin is slurred in water. The slurry is added to the column and backwashed with water to eliminate air pocket and distribute the beads.
Acid, typically 0.1N HCl is added to convert the acid form (H+ ion) followed by washing with water. Then a salt solution of drug is added, followed by rewashing with water. The cake is removed from column, filtered and oven dried. An analogous procedure can be used to adsorb a carboxylated drug on an anion exchange resin, using NaOH to convert the resin to the basic form (Na+ ion).
2.6.9 Microencapsulation of Resinate12,13:
The approach is used to control or sustain the release of drug. It acts as a rate limiting barrier for the entry of the ions into the resinate and also for the release of drug from the microcapsules.
Types:
1. Emulsion Polymerization: According to this technique the monomer (alkyl acrylates) is added drop wise to the stirred aqueous polymerization medium containing the material to be encapsulated (core material) and a suitable emulsifier. As the polymerization proceeds, these nuclei grow gradually and simultaneously entrap the core material to form the final microcapsules.
2. Suspension cross linking: Microcapsule formation by this technique involves dispersion of an aqueous solution of the polymer containing core material in an immiscible organic solvent (suspension/dispersion medium) in the form of small droplets. The droplets are subsequently hardened by covalent cross linking and are directly converted to the corresponding microcapsules.
3. Solvent evaporation/extraction: The polymer is dissolved in a water immiscible volatile organic solvent like dichloromethane or chloroform, into which the core material is also dissolved or dispersed. The resulting solution is added drop wise to a stirring aqueous solution having a suitable stabilizer like poly (vinyl alcohol) or polyvinyl pyrrolidone, etc. to form small polymer droplets containing encapsulated material. With time, the droplets are hardened to produce the corresponding polymer microcapsules.
4. Coacervation/phase separation: Phase separation processes are divided into simple and complex Coacervation. Simple Coacervation involves the use of a single polymer such as gelatin or ethyl cellulose, in aqueous or organic media, respectively. Complex Coacervation involves two oppositely charged polymeric materials such as gelatin and acacia, both of which are soluble in aqueous media. In both the cases, Coacervation is brought about by gradual desolvation of the fully solvated polymer molecules. The Coacervation mixture is cooled to about 5-20 °C, followed by the addition of a cross linking agent to harden the microcapsule wall formed around the core particles.
2.6.10 Characterization of Resinate Microcapsules12:
Table V: Evaluation methods of Resinate microcapsules
|
Sr no. |
Characteristics |
Evaluation method |
|
1. |
Microscopy |
SEM |
|
2. |
Particle Size and Distribution |
Laser Diffraction Particle Size Analyzer |
|
3. |
Coating Polymer on microcapsules |
Percentage Coating = Microcapsules weight. – dried complex weight Microcapsules weight |
|
4. |
Drug content |
Extraction of microcapsules by centrifugation |
|
5. |
In Vitro drug release |
Dissolution in simulated intestinal fluid |
Drug release of the drug-resin complex and its microcapsules14:
It is controlled by three possible mechanisms
(1) Mass or chemical reaction control: The exchange reaction between the counter ion and drug.
(2) Particle diffusion control: The release of drug through the porous within its particles.
(3) Membrane diffusion control: The release of drug across the thin layer around the particle.
2.7 MARKETED ION EXCHANGE RESINS:
The popular resin brands available in the market:
Table VI: Various brands and manufacturer of IER
|
Sr.no |
Brand |
Manufacturer |
|
1. |
DOWEX |
The Dow Chemical Company |
|
2. |
AMBERLITE |
Rohm and Haas Company |
|
3. |
INDION |
Ion Exchange India Pvt. Ltd |
|
4. |
TULSION |
Thermax India Pvt. Ltd |
2.7.1 Dowex15:
The nomenclature used to identify DOWEX™ fine mesh resins is designed to communicate specific information regarding resin type and degree of cross-linkage.
There are three resin types: strong acid cation exchange resins designated as “50W”, Type I strong base anion exchange resins designated as “1”, and Type II strong base anion resins designated as “2”.
Under the Dow nomenclature, one of these three designations appears in conjunction with an “X” number describing the degree of resin cross linkage (the X number is the percentage of divinyl benzene DVB in the resin copolymer). Therefore,
DOWEX 50WX8 is a strong acid cation resin containing 8% DVB.
DOWEX 1X4 is a Type I strong base anion resin containing 4% DVB.
Table VII: Dowex Cation and Anion Exchange Resin
|
CATION RESIN |
Mesh Size |
Ionic form |
Water retention capacity % |
Total exchange capacity(meq/mL) |
|
Dowex 50WX2 |
50-100 |
H+ |
74-82 |
0.6 |
|
Dowex 50WX2 |
100-200 |
H+ |
74-82 |
0.6 |
|
Dowex 50WX2 |
200-400 |
H+ |
74-82 |
0.6 |
|
Dowex 50WX4 |
50-100 |
H+ |
64-72 |
1.1 |
|
Dowex 50WX4 |
100-200 |
H+ |
64-72 |
1.1 |
|
Dowex 50WX4 |
200-400 |
H+ |
64-72 |
1.1 |
|
Dowex 50WX8 |
50-100 |
H+ |
50-56 |
1.7 |
|
Dowex 50WX8 |
100-200 |
H+ |
50-56 |
1.7 |
|
Dowex 50WX8 |
200-400 |
H+ |
50-56 |
1.7 |
|
ANION RESIN |
|
|
|
|
|
Dowex 1X2 |
50-100 |
Cl- |
65-75 |
0.7 |
|
Dowex 1X2 |
100-200 |
Cl- |
70-80 |
0.6 |
|
Dowex 1X2 |
200-400 |
Cl- |
70-80 |
0.6 |
|
Dowex 1X4 |
50-100 |
Cl- |
50min |
1.0 |
|
Dowex 1X4 |
100-200 |
Cl- |
55-63 |
1.0 |
|
Dowex 1X4 |
200-400 |
Cl- |
55-63 |
1.0 |
|
Dowex 1X8 |
50-100 |
Cl- |
43-48 |
1.2 |
|
Dowex 1X8 |
100-200 |
Cl- |
39-45 |
1.2 |
|
Dowex 1X8 |
200-400 |
Cl- |
39-45 |
1.2 |
2.7.2 Amberlite16:
Table VIII: Brands of AMBERLITE
|
Commercial name |
Compendia name |
Matrix type |
Nature |
Ionic form |
Application |
|
AMBERLITE™ IRP64 |
Polacrilex Resin |
Methacrylic acid divinyl benzene polymer |
Weak acid |
H+ |
• Taste masking agent • Drug stabilizing agent • Nicotine |
|
AMBERLITE™ IRP88 |
Polacrilin Potassium |
Methacrylic acid divinyl benzene polymer |
Weak acid |
K+ |
• Tab. disintegrant • Taste masking agent |
|
AMBERLITE™ IRP69 |
Sodium Polystyrene Sulfonate |
Styrene-divinyl benzene polymer |
Strong acid |
Na+ |
• Sustained release • Drug stabilizing agent • Taste masking agent |
|
DUOLITE™ AP143 |
Cholestyramine |
Styrene-divinyl benzene |
Strong base |
Cl- |
• Sustained release • Drug stabilizing agent • Taste masking agent |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Indion17:
Table IX: Brands of INDION
|
Commercial Name |
Matrix type |
Particle size |
Functional group |
Total exchange capacity (meq/mL) |
Ionic form |
Application |
|
INDION 224 |
Styrene Divinyl benzene |
0.2 - 12 |
- SO3 |
4.8 |
H+ |
Sustained release agent |
|
INDION 244 |
Styrene Divinyl benzene |
≤0.15 |
- SO3 |
4.5 |
H+ |
Sustained release agent |
|
INDION 254 |
Sod. Polystyrene Sulfonate USP |
≤0.15 |
- SO3 |
NA |
Na+ |
Sustained release agent, USP specified |
|
INDION 284 |
Styrene Divinyl benzene |
0.3 – 1.2 |
- SO3 |
1.0 |
Na+ |
Sustained release agent |
1.1.1 Tulsion18:
Table X: Brands of TULSION
|
Commercial name |
Compendia name |
Application |
|
Tulsion®335 |
Polacrilex |
· Taste masking · Preparation of nicotine Polacrilex · Vitamin b 12 stabilization |
|
Tulsion®339 |
Polacrilex Potassium USP |
· High performance tablet disintegrant · Taste masking |
|
Tulsion®344 |
Sodium Polystyrene Sulphonate USP |
· Sustained release or Modified release agent · Drug stabilization · Taste masking |
|
Tulsion®345 |
Calcium Polystyrene Sulphonate BP |
· Treatment of blood disorders · Potassium reduction in blood |
|
Tulsion®142(CHL) |
Cholestyramine Resin USP |
· Reduction of bile acids Cholesterol reduction · Taste masking |
3 Resinates: Work Reported:
The reported work of resinates for various drug delivery system is summarized in table XI.
Table XI: Reported work of Resinate
|
Sr.no |
Drug |
Resin |
Polymer |
Type of system |
Ref. |
|
1. |
Amoxicillin trihydrate |
Dowex 1-x4 and Dowex 1-x8 |
Carbopol934, Poly carbophil |
oil-in-oil solvent evaporation technique, mucoadhesive microparticles |
19 |
|
2. |
Chlorpheniramine Maleate, Pseudo ephedrine HCL and Propranolol HCL |
Amberlite ® IRP 69 |
Ethyl cellulose, Poly(methyl methacrylate), Eudragit RS 100 |
microencapsulated with an aqueous solvent evaporation method, |
20 |
|
3. |
Chlor pheniramine maleate |
Indion 244 |
Eudragit RS 100 |
Solvent evaporation technique, microcapsules in sustained release suspensions |
21 |
|
4. |
Ciprofloxacin |
Indion 234 |
Poly ethylene glycol |
PEG treatment of DRC successfully retards ciprofloxacin ion exchange release in acidic pH |
22 |
|
5. |
Ciprofloxacin hydrochloride I.P |
Indion® 254F |
Carbopol ® 980NF and Hydroxy Propyl methyl cellulose |
Once a day ophthalmic delivery system for Based on the concept of pH-triggered in situ gelation. The in situ gelling system involves the use of polyacrylic acid (Carbopol®980NF) as a phase transition polymer, HPMC(Methocel ® K100LV) as a release retardant, and ion exchange resin as a complexing agent. |
23 |
|
6. |
Dextromethorphan |
|
Ethyl cellulose and poly(vinyl acetate) (Kollicoat® R30D) |
Sustained release fast disintegrating tablets (FDTs), coated particles were granulated with suitable tablet excipients and then compressed into the tablets. |
24 |
|
7. |
Dextromethorphan hydrobromide |
Amberlite® IRP69 |
Aquacoat ECD, Surelease®,KollicoatSR® 30D, Poly vinyl acetate; Ethyl cellulose |
Fuidized bed coater, sustained release fast-disintegrating tablets |
25 |
|
8. |
Dextromethorphan hydrobromide |
Amberlite® IRP69 Dowex® 50W×4 Dowex® 50W×8 |
Ion Exchange resin |
Simple resinate of drug |
26 |
|
9. |
Dextromethorphan hydrobromide |
Dowex® 50W×4
|
Microcrystalline cellulose (AvicelPH102), |
Multiple-Unit Dextromethorphan Resinate Tablets, |
27 |
|
10. |
Dextromethorphan hydrobromide |
Dowex® 50WX4-200 |
Kollicoat® SR 30D |
Polymer coated pellets |
28 |
|
11. |
Diclofenac sodium |
Dowex1-X2 |
Aquacoat® or Eudragit® RS30D |
Microcapsules by Wurster Process |
08 |
|
12. |
Diclofenac sodium |
Dowex 1-x4 and Dowex 1-x8 |
Hydroxy Propyl methylcellulose phthalate |
Microencapsulated by non-aqueous emulsion solvent evaporation method |
29 |
|
13. |
Diclofenac sodium |
Duolite ATP-143 |
Simple resinates |
Hydroxy Propyl methyl cellulose (HPMC) matrix tablets to modify the release of oppositely charged drugs. Upon contact with the dissolution mEdnum, a gel layer formed rapidly around the solid tablet core
|
30 |
|
14. |
Diltiazem |
Dowex® 50W×4 Dowex® 50W×8 |
Cellulose acetate butyrate(CAB), , PVA |
Microcapsules in sustained release suspensions |
12 |
|
15. |
Diltiazem HCl |
Indion254 |
Polystyrene, Methylcellulose |
Oil-in-water Emulsion Solvent Evaporation method, drug release from the microencapsulated resinate followed the diffusion controlled model in accordance with the Higuchi equation. |
31 |
|
16. |
Diphenhydramine Hydrochloride |
Amberlite IRP-69 |
Methocel K4M (HPMC) or Ethocel 7cP (EC |
The incorporated resin differently influenced DPH release from HPMC- and EC-based matrices in deionized water. The resin further retarded DPH release from HPMC-based matrices due to the gelling property of HPMC. |
32 |
|
17. |
Naproxen Sodium |
Amberlite IP69, Amberlite IP88, Duolite AP143 |
Ethyl cellulose and Eudragit RL |
Matrices prepared by using drug resin complex |
33 |
|
18. |
Nicotine |
AmberliteIR-120, Dowex 50W ×2 Dowex® 50W×4 Dowex® 50W×8 |
4% agar solution |
Hydrogel, of resinate |
34 |
|
19. |
Phenyl propanolamine |
Cation- exchange resin |
cellulose acetate butyrate |
Drug-resin particles were coated with cellulose acetate butyrate using an emulsion-solvent evaporation technique. |
35 |
|
20. |
Phenyl propanolamine, Chlorpheniramine, |
Amberlite® IR-120 Amberlite® XE-69, |
Ethyl cellulose |
Fluidized bed coating to the resinate particles |
36 |
|
21. |
Phenyl propanolamine |
Amberlite® IR-120 or Amberlite® XE-69, |
Ethyl cellulose |
Pretreatment of the ion-exchange resin-drug complex particles with an agent such as polyethylene glycol, application of an atomized polymer solution to the fluidized ion-exchange resin-drug complex particles |
10 |
|
22. |
Propranolol hydrochloride |
Amberlite XE-364R, IRP-69, IR-120 PLUS, IR-122 |
|
Simple resinates were formed by Batch method |
37 |
|
23. |
Propranolol HCL, Diclofenac sodium, Guaifenesin |
Amberlite IRP69, Amberlite IRP88, Duolite ATP143, Dowex 50WX8 |
Hydroxy Propyl methyl cellulose E4M,K4M,K15M and K100M |
Matrix tablets of polymer |
30 |
|
24. |
propranolol hydrochloride |
Indion 254 |
Polystyrene |
Microencapsulated with polystyrene using an oil-in-water emulsion-solvent evaporation method |
38 |
|
25. |
Propranolol–HCl |
Indion254® |
Poly ethylene imines |
Drug-loaded calcium alginate beads, |
39 |
|
26. |
Ranitidine Hydrochloride |
Amberlite IRP 69 |
Simple resinates |
Taste masking as well as sustaining the release of Ranitidine hydrochloride. |
40 |
|
27. |
Sulfadiazine sodium |
Dowex 1-X8 |
Ion Exchange resin |
Simple resinate by W/O/W emulsion evaporation technique, |
41 |
|
28. |
Terbutaline hemisulphate |
Dowex® 50W-x4, |
Eudragit ® RS and RL, silicon, PVA |
O/O and O/W microencapsulated suspension |
42 |
|
29. |
Theophylline |
Dowex 2X10 |
Eudragit RS |
Coacervation phase separation technique |
43 |
|
30. |
Tramadol |
Cation-exchange resin, Cross linkage 8 |
Ethyl cellulose (EC) of various viscosities |
Microencapsulation of tramadol–resin complexes (TRC) was carried out by the spray-drying method, |
44 |
|
31. |
Venlafaxine hydrochloride |
Amberlite® IRP69 |
Eudragit® RS100 and Eudragit® RL100 |
PEG 400 treatment with the resinates. Microencapsulation is done by emulsion solvent diffusion method. |
45 |
|
32. |
Verapamil HCl |
Indion 244 Indion 254 |
HPMC |
Resinates are formulated as pellets with hydroxyl propyl methylcellulose by extrusion-spheronization |
46 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4. Resinates: Patented Work
The table XII summarizes the patented work of resinates.
Table XII: Patented work of Resinate
|
Sr.no |
Patent.no |
Drug |
Resin |
Polymer |
Type of system |
Ref |
|
1. |
EP 0429732. |
Betaxolol |
Amberlite ® IRP 69 |
CARBOPOL-934 P (Carbomer) |
Formulation 1.) gels; 2.)pourable liquids; 3.) anhydrous salts |
47 |
|
2. |
USP 6001392 |
Dextromethorphan hydrobromide |
Amberlite ® IRP 69, Dowex |
Ethyl cellulose, Ethyl cellulose latex, SURELEASE |
Sustained release antitussive preparation |
48 |
|
3. |
USP 4931279 |
Pilocarpine hydrochloride |
carboxylated styrene cross-linked by divinyl benzene |
|
Hydrogel Polymer Containing an Ion-Exchange Resin, Preparation of contact lens |
49 |
|
4. |
USP 7,001,615 |
basic active means a positively-charged, ophthalmically, otically or nasally acceptable active agent |
Amberlite Dowex |
Polymeric Suspending Component: HPC. CMC,HPMC,HEC,Na-CMC,Ca-CMC CARBOPOL |
The polymeric suspending component consists essentially of a combination of a carboxy vinyl polymer and a polymer |
50 |
|
5. |
US Patent Application 20070215511 |
Morphine, Albuterol, Methylphenidate, Dextromethorphan, CodeinePO4, Tramadol, Pseudoephedrine, Phenylephrine , Venlafaxine , Oxybutynin , Metformin , Ibuprofen, |
Amberlite IRP-69 and Dow XYS-40010.00 |
KOLLICOAT SR 30D, AQUACOAT™ ECD-30 or SURELEASE™, Eudragit: RS, RL30D, RL100, or NE |
Final dosage form was Suspension |
51 |
|
6. |
USP 4788055 |
Dextromethorphan HBr |
Amberlite IRP 69 |
|
liquid sustained release Antitussive formulation |
52 |
|
7. |
USP 4996047 |
Pseudoephedrine, Phenyl propanolamine, Dextromethorphan, buprofen, |
Amberlite IRP-69, Dow XYS-40010.00 |
Ethyl cellulose |
fluid-bed coating, hard shell gelatin capsule, tablet, chewable tablet,and Suspension for oral administration was formulated |
53 |
|
8. |
USP 4999189, USP 5186930 |
Pseudoephedrine Free Base |
Dowex 50 WX8 |
Ethyl cellulose, Wax |
Wax coating to the Polymer and then spray coating method employing bottom spray fluidized bed equipment is used. |
54, 55 |
|
9. |
USP Application 20050265955 |
Hydrocodone bitartrate |
DOWEX 50WX8H, AMBERLITE IRP-69 |
Ethyl cellulose (AQUACOAT), (SURELEASE), |
Sustained release drug particles comprise a drug- ion exchange resin complex and a water-permeable diffusion barrier. Coating by laboratory-scale fluid bed processor. |
56 |
|
10. |
Patent Application 20080118570 |
Dextromethorphan, Oxybutynin, Hydrocodone, Venlafaxine, Metoprolol |
Amberlite® IRP69 |
Eudragit® RS, Eudragit® RL, Eudragit® NS |
Polyethylene Glycol Impregnation of Drug Resin Complex, Uni-Glatt processor is used to coat by polymers. Final formulation was the suspension |
57 |
|
11. |
Patent Application 20050181050 |
Dextromethorphan HBr, monohydrate |
Amberlite IRP-69 |
Eudragit RS 30 D |
Wurster Column is used for coating purposes, Extended Release Liquid Composition |
58 |
|
12. |
Patent Application 20070140983 |
Dextromethorphan HBr |
AMBERLITE IRP69 |
Ethyl cellulose |
fluid-bed coating apparatus |
59 |
|
13. |
Patent Application 20030099711 |
Phenyl propanolamine, Dextromethorphan |
|
Ethyl cellulose, Surelease® |
coated with an aqueous dispersion of ethyl cellulose using a Wurster coating system |
60 |
Conclusion:
The ion exchange resin (IER) is the multifunctional excipients having a wide application i.e. taste masking, sustained release, controlled release, tablet disintegrant, improvement of dissolution of poorly soluble drugs, and stability of Vitamins etc. IER in drug delivery research is gaining importance and commercial success as evidenced by the number of patents and technological developments. With the advent of various drug delivery approaches for example site-specific, transdermal, nasal and ophthalmic, floating, pH and ionic strength-responsive systems IER would no doubt be the first choice excipient. The review article is an insight to explore the potential of IER in developing new formulations for a variety of drugs for high commercial outputs in the near future.
References:
1. Ranade V V, Hollinger M A., Drug Delivery Systems, CRC Press LLC,Second Edntion, 28-29, (2004).
2. K.K. Jain, Drug Delivery Systems, In: S.B. Tiwari and Ali R. Rajabi-Siahboomipg (Eds), Extended-Release Oral Drug Delivery Technologies: Monolithic Matrix Systems, Humana Press, 2008, 218.
3. Banker G S and Rhodes C T, Modern Pharmaceutics, Marcel Dekker Pub. Sustained and Controlled Drug Delivery System, 2nd Edn. pp.635-638.
4. XIII-Water-D-Ion Exchange Resins Article
5. Mrs. Ashwini R. Madgulkar, Ion Exchange Resins in Formulation: An Update, pharmainfo.net, in Latest Reviews, 5, 1, (2007).
6. Anand V, Kandarapu R And Garg S, Ion-Exchange Resins: Carrying Drug Delivery Forward, Reviews Research Focus, Drug Delivery Tech, 6, 17, (2001).
7. Lyn Hughes global technical service manager, healthcare process solutions at Rohm and Haas Company, Pharmaceutical Technology Europe,17(4), 38–42, (2005).
8. Hideki I, Kazuhiro F, Yoshinobu F, Use of Ion Exchange Resins to prepare 100um sized microcapsules with Prolonged drug Release by the Wurster process, Int. J. Pharm. Sci., 216, 67-76, (2001).
9. Y Raghunathan, Prolonged release pharmaceutical preparation, US Patent 4,221,778, 2001.
10. Raghunathan Y and et al, Sustained-release drug delivery system I: Coated ion-exchange resin system for phenyl propanolamine and other drugs, J. Pharm. Sci. 70(4), 379-84, (1981).
11. Husain M A et al Hollow fibers as an oral sustained release delivery system, Pharm. Res. 6, 49-52, (1989).
12. Junyaprasert V B, Manwiwattanakul G Release pro?le comparison and stability of diltiazem–resin microcapsules in sustained release suspensions, Intr. J. Pharm. 352, 81–91, (2008).
13. Dubey R, Shami T C and Bhasker Rao K U, Microencapsulation Technology and Applications, Defence Sci. J. 59, 82-95, (2009).
14. Gyselinck,P., Sueyaert,H., VanSeveren,R., Brackman,P., Drug–polymer combinations.Part2. Evaluation of some mathematics approach to drug release from resinate. Pharmazie. 37 190–192.
15. DOWEX™ Fine Mesh Spherical Ion Exchange Resin, Form No. 177-01509-904, http://www.dow.com/liquidseps/prod/dx_50wx4.htm
16. ROHM and HASS, Advanced Release Technology, http://www.rohmhaas.com/ionexchange/pharmaceuticalsHEALTHCARE©ROHM
17. Ion Exchange India, Pvt. Ltd.)www.ionexchange.com
18. http://www.thermaxindia.com
19. Cun Äa M et al., Preparation and in vivo evaluation of mucoadhesive microparticles containing amoxicillin-resin complexes for drug delivery to the gastric mucosa, Eur. J. Pharm. Biopharm. 51, 199-205, (2001).
20. Sriwongjanya M, Bodmeier R, Entrapment of drug-loaded ion-exchange particles within polymeric microparticles, Intr. J. Pharm. 158, 29-38, (1997).
21. Kadam A U, Sakarkar D M, Kawtikwar P S, Oral Controlled Release Chlorpheniramine-Ion Exchange Resinate Suspension, Ind. J. Pharm. Sci. 70 (4), 531-534, (2008).
22. Pisal S, Zainnuddin P, Nalawade P, Mahadik K, Kadam S, Drug release properties of polyethylene-glycol-treated ciprofloxacin-Indion 234 complexes, AAPS Pharm. Sci. Tech. 5 (4), 101-106, (2004).
23. Jain, Satishkumar P., Shah, Sejal P., Rajadhyaksha, Namita S., Pirthi, Pal Singh P. S. and Amin, Purnima D. 'In Situ Ophthalmic Gel of Ciprofloxacin Hydrochloride for Once a Day Sustained Delivery', Drug Dev. and Ind. Pharm. 34:4, 445 – 452, (2008).
24. Jeong S H, Park K, Development of sustained release fast-disintegrating tablets using Various polymer-coated ion-exchange resin complexes , Int. J. Pharm. 353, 195–204, (2008).
25. Jeong S H, Park K, Drug loading and release properties of ion-exchange resin complexes as a drug delivery matrix, Int. J. Pharm.361, 26–32, (2008).
26. Pongjanyakul T, Prakongpanb T S, Rungsardthonga U, Chanchama P, Priprem A, Characteristics and in vitro release of Dextromethorphan resinates, Powder Technology, 152, 100–106, (2005).
27. Pongjanyakul T and et al, Effect of Sampling Procedures of Release Testing on Drug Release and Scale-up Production Feasibility of Multiple-Unit Dextromethorphan Resinate Tablets :A Technical Note, AAPS Pharm. Sci. Tech. 8(4), 117, (2007).
28. Berhane N H, Jeong S H, Haghighi K, Park K, Modeling film-coat non-uniformity in polymer coated pellets: A stochastic approach, Int. J. of Pharm. 323 1(2), 64-71, (2006).
29. Torres D, Garcla-Encina G, Seijo B, Vila J L, Jato, Formulation and in vitro evaluation of HPMCP-microencapsulated drug-resin complexes for sustained release of diclofenac, Int. J. Pharm. 121, 239-243, (1995).
30. Sriwongjanya M, Bodmeier R, Effect of ion exchange resins on the drug release from matrix tablets, European Journal of Pharmaceutics and Biopharmaceutics, 46, 321–327, (1998).
31. Halder A and Biswanath Sa, Preparation and In Vitro Evaluation of Polystyrene Coated Diltiazem-Resin Complex by Oil-in-Water Emulsion Solvent Evaporation Method, AAPS Pharm. Sci. Tech. 7(2), 46, (2006).
32. Akkaramongkolporn,T, Ngawhirunpat P, Nunthanid,J, Opanasopit P, Effect of a Pharmaceutical Cationic Exchange Resin on the Properties of Controlled Release Diphenhydramine Hydrochloride Matrices Using Methocel K4M or Ethocel 7cP as Matrix Formers, AAPS Pharm. Sci. Tech. 9(3), 899-908, (Sep 2008).
33. Melisa Martinez, Evone S. Ghaly, Sustained Release Naproxen Sodium Matrices Prepared by Ion Exchange Resin and Polymer, http://www.aapsj.org/abstracts/AM_2001/134
34. Conaghey O M , Corish J, Corrigan O I, The release of nicotine from a hydrogel containing ion exchange resins, Int. J. Pharm.170, 215–224, (1998).
35. Omar L. Sprockel, Prapaitrakul W, Effect of eluant properties on drug release from cellulose acetate butyrate-coated drug resin complexes, Int. J. Pharm. 48(1-3), 217-222, (1988).
36. Hall H S, Sustained Release From Coated Ion Exchange Resins by Coating Place, Inc.Verona, WI 53593.
37. Burke, Gerald M., Mendes, Robert W. and Jambhekar, Sunil S. 'Investigation of the Applicability of Ion Exchange Resins as a Sustained Release Drug Delivery System For propranolol Hydrochloride', Drug Dev. and Ind. Pharm. 12:5, 713 – 732, (1986).
38. Halder A, Sa B, Halder A, Sa B, Sustained release of propranolol hydrochloride based on ion-exchange resin, J Microencapsul. 23(8), 899-911, (2006).
39. Halder A et al., Entrapment efficiency and release characteristics of Polyethyleneimine -treated or-untreated calcium alginate beads loaded with propranolol–resin complex, Inter. J. Pharm. 302, 84–94, (2005).
40. Khan S and Guha A and et al,Strong cation exchange resin for improving physicochemical properties and Sustaining release of Ranitidine Hydrochloride, Ind. J. Pharm. Sci. 69 (5), 626-630, (2007).
41. Kondo T, Hafez E, Abdel-Monem H, Muramatsu N, Haras S E, EI-Gibaly I, Preparation and evaluation of microencapsulated sulfadiazine resin complex, Powder Tech. 88, 101-105, (1996).
42. Cuna M, Vila Jato J L, Torres D, Controlled-release liquid suspensions based on ion-exchange Particles entrapped within acrylic microcapsules, Inter. J. Pharm.199, 151–158, (2000).
43. Atyabi F, Sharma H L , Mohammad H A H, Fell J T, Controlled drug release from coated floating ion exchange resin beads, J. Cont. Rel. 42, 25-28, (1996).
44. Zhang Z Y, Ping Q N, Xiao B, Microencapsulation and characterization of Tramadol–resin complexes, J. of Controlled Release, 66(2-3), 107-113, (2000).
45. Liu HF, Zhang CY and et al, Study of the formulation parameters affecting the preparation of microencapsulated ion-exchange resins containing venlafaxine hydrochloride, Ind. J Pharm. Sci. 69 (4), 550-555, (2007).
46. Bhalekar MR, Avari J, Umalkar RA,Preparation and in vitro evaluation of sustained release drug delivery system for Verapamil HCL, Ind. J Pharm. Sci. 69 (3), 418-42, (2007).
47. Jani, Rajni, Harris, Robert Gregg, Sustained-release compositions containing cation exchange resins and poly carboxylic polymers, EP0429732, 1994.
48. Antitussive drug delivery by ion exchange resin. US6001392, 1999.
49. Bawa, Rajan, Ruscio, Dominic V., Sustained release formulation containing an ion-exchange resin, US 4931279, 1990.
50. Singh; Onkar N, Sustained release ophthalmic, otic and nasal suspension, US7001615, 2006.
51. Mehta, Ketan, Tu, Yu-hsing, Modified release formulations containing drug-ion exchange resin complexes, US Patent Application 20070215511, 2007.
52. Fischer X, Franz, Khanna, Satish C., Resinate sustained release Dextromethorphan composition, US4788055, 1988.
53. Kelleher, William J., Carpanzano, Anthony E., Sustained release drug-resin complexes, US4996047, 1991.
54. Kogan, Patricia W., Rudnic, Edward M., Sequeira, Joel A.,Chaudry, Imtiaz A , Sustained release oral suspensions, US4999189, 1991.
55. Kogan, Patricia W., Rudnic, Edward M., Sequeira, Joel A.,Chaudry, Imtiaz A, Sustained release oral suspensions, US5186930, 1991.
56. Raman, Siva N., Cunningham, John P., Lang, John F , Sustained release preparations, US Patent Application 20050265955, 2005.
57. Zhi Liu, Bei Chen, Danchen Gao, Mukti S. Rao, Arunya Usayapant, Polymer Coated Drug-Ion Exchange Resins And Methods, US Patent Application 20080118570, 2008.
58. Hirsh, Jane, Fleming, Alison B., Rariy, Roman, Dosage forms using drug-loaded ion exchange resins, US Patent Application 20050181050, 2005.
59. Hall, Harlan, Madsen, Scott J., Ion Exchange Resin Treated to Control Swelling, US Patent Application 20070140983, 2007.
60. Meadows, David, Young, Peter, Keyser, Donald J, Sustained release preparations, US Patent Application 20030099711, 2003.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









