 About Authors:
About Authors:
Bharathi.V*, SwarnaLatha.D, M.Sreenivasulu
Annamcharya college of pharmacy, newboyana pally, rajampet(M),
kadapa(dt). Andhara Pradesh, India.
*bharu.v.net@gmail.com
ABSTRACT:
Medicinal plants have played an essential role in the development of human culture. Many of the modern medicines are produced indirectly from medicinal plants, for example aspirin. The present study was concentrated on past work reported on the some mussaenda species and study of different activities and phyto constitutions reported on mussaenda species. The genus Mussaenda (rubiaceae) is an important source of medicinal natural products, steroids, flavonoids, glycosides and only a few number of species reported positive for alkaloids and tannins. Many Mussaenda species were reported to possess anti-oxidant, anti-inflammatory in different models, analgesic, antimicrobial, diuretic, antiphlogistic and antipyretic, acute gastroenteritis and dysentery, anti- fertility activity, antiviral property , antibacterial effect rarely for hepato protective activity and wound healing activity.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1613
INTRODUCTION OF MUSSAENDA SPECIES:
Mussaendasare increasingly popular for the showy color they provide during much of the year in South Florida landscapes(14). The mussaendas are a group of highly ornamental shrubs suited to tropical and subtropical climates with a bright future, both as landscape plants and as potted floral decorations(14). Some species of Mussaenda have been used in Chinese and Fijian traditional medicine.
They're members of the large Rubiaceae family, which also contains Gardenia, Ixora, Pentas and Coffea (coffee).
The most distinctive feature of Mussaenda (and some other genera of the Rubiaceae) is that the floral display is primarily derived from the calyx, with some individual flowers within an inflorescence carrying an enlarged petaloid sepal. Some cultivars have all five sepals enlarged. These are called calycophylls or sometimes semaphylls (that is, a structure which signals a pollinator). In many publications, these are erroneously referred to as bracts.
There are Mussaenda species native to Africa, Madagascar, Asia and the Pacific. Commonly cultivated species include Mussaendaphilippica, M. erythrophylla, M. frondosa and Pseudomussaendaflava (also referred to as Mussaendaflava, M. glabra, M. luteola, M. lutea or M. incana in various publications).
University of the Philippines Los Baños has been active over many decades in breeding these ornamentals and is responsible for the popular cultivars 'Queen Sirikit', 'Doña Aurora' and 'Doña Luz'. Many more cultivars have been developed in the Philippines, although not widely available in Australia.
Many are named after First Ladies and other notable women of the Philippines, hence the Spanish form of address "Dona" in some cultivar names. 'Queen Skirit' was named after the Queen of Thailand to commemorate a visit to the Philippines.
The major attractionsof mussaendas in the landscape is their extended flowering period. They will loosen their leaves and go dormant through the cooler and drier winter, but put on a spectacular display throughout the warm, wet months. If conditions are suitable, they can flower year-round. They have poor drought and cold tolerance.
[adsense:468x15:2204050025]
External characters:
Herbaceous but woody in the lower portion, erect, cylindrical, branched, differentiating in the nodes and internodes, internodes are hairly, ramal, cauline, simple, opposite decussant, petiolate, interpetiolate stipules are present, ovate, margin entire, apex acute, unicostatereculate venation, dichasialcyame. Bracteates, pedicellate, complete hermaphrodite, action morphine but mature and older flowers are zygomorphic, pentamerous, epigynous & yellow in color. Sepals five, poly sepalous one sepal is slightly larger than the remaining four. All sepals are same shape and color persistant, valvate aestivation rose color. Petal five gamopetalous, corolla tube elongated funnel shaped, valvate aestivation, yellow in color, coronary structures are present in the form of silky hairs. Stamens five polyandrous, epipetalous, dithecous, basifixed, introrse. Bicarpellary, syncarpous, inferior binocular, may ovules, axilepalcentation, style long with two stigmatic lobes.
In addition to their role in the garden, mussaendas have potential as potted floral gifts. Research in the Phillpines investigating appropriate treatment including growth regulators and selection of suitable hybrids is aimed at developing this market. Given that Mussaendas can bloom year-round in suitable climates, there is presumably no daylength requirement. This gives them a particular advantage over Poinsettia as a floral Christmas decoration in parts of the world where flowering must be artificially induced.
As research and hybridisation work progresses, cultivars with new colours, growth habits, climatic tolerances and amenability to propagation could mean that we'll be seeing more of these these flamboyant shrubs in our lives in the future.
Phytochemical constituents reported from Mussaendaspecies:
The phytochemistry of Mussaenda species has been studied extensively since 1990s. Iridoids, flavonoids and triterpenes are the common chemical ingredients distributed in Mussaenda species. The most recognized compounds in Mussaendas are the iridoids and triterpenesaponins. Iridoid glycosides, Mussaenoside and shanzhiside methyl esterhave been reported from Mussaendaparviflora and Mussaendashikokiana. (18)
The leaves of M. arcuatayield Astragalin,isoquercitrin, kaempferol-3-O-beta-Drutinoside and the two phenylpropanoid derivatives as melilotoside and dihydromelilotoside(09) Mussaendosides M and N are the saponins from M.pubescens(07). Mussaein A, Mussaein B and MussaeinC (12) are the monoterpenes from M.pubesens.
Mussaendapubescensis reported to contain several triterpenes and triterpenoidsaponins namely mussaendosides U, V, M, O, P and Q(13). M.macrophylla afforded Mussaendoside W 7(08). A new compound Sanzhilactonealong with mussaenoside, barlerin, lupeol and beta-D-glucose has been obtained from the stem of M. incana(01) . Quercetin, rutin, hyperin, ferulic acid, sinapic acid, beta sitosterol, saponin occurs in M.raiatensi(15)
Aureusidin(29)iridoid glycosides(18) two novel triterpenoidsaponins, named mussaendosides U(1) & V(2) (28)mussaendosides A- C ; M & N with cyclolanostene type aglucone(26,27) mussaendosides G(1) & K(2) two new triterpenoidsaponin(12) and three monoterpenes from M.pubescenswere reported .
Pharmacology
MussaendapubescensAit.f It has been used in Chinese folk medicine as a diuretic, antiphlogistic and antipyretic.
· It is also used to detoxify mushroom poisons and terminate early pregnancy(02,03).
M. macrophylla Triterpene glycosides from the stem barhas been shown to be active against oral pathogens (08).
M. frondosa has been found to possess antibacterial effect (05).
Mussaendaphillipica The sepals of cultivars are active (19).
· Sanshiside methyl ester posssess antiviral property (10).
· Non glycosidiciridoids like Mussaein are cytotoxic (06).
Mussaendapubescens exhibited anti-RSV activity with 50% inhibition. (16).
Mussaendaerythrophylla Diuretic, antiphlogistic, antipyretic and effective in laryngopharyngitis, acute gastroenteritis and dysentery(02) and also anti- fertility activity.
Some species of Mussaendahave been used in Chinese and Fijian traditional medicine.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Few Structures of Phytoconstituents isolated from mussaenda species:
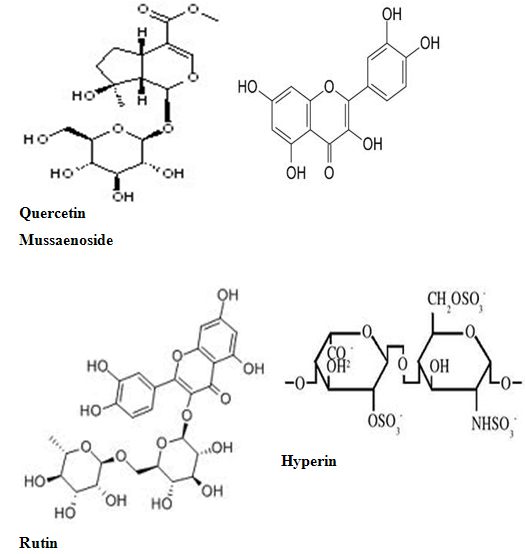
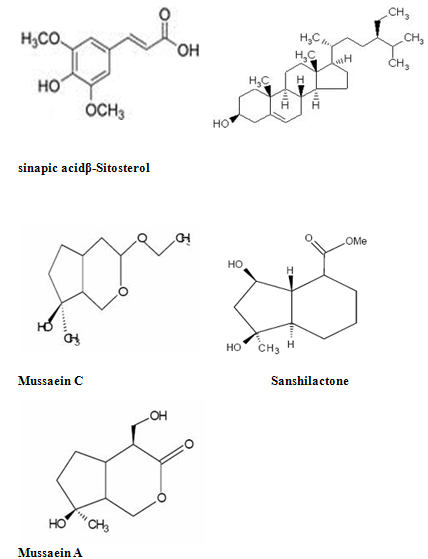
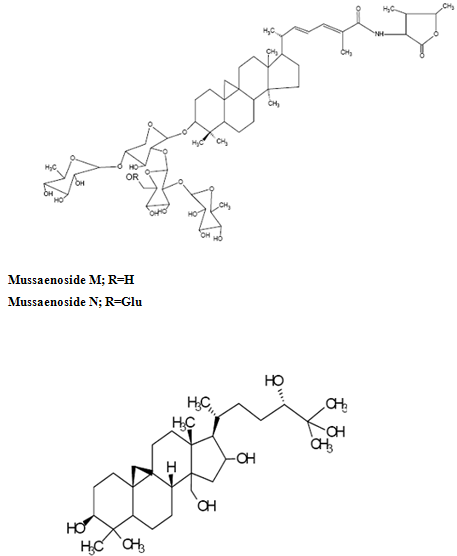
Mussaendoside W
INTRODUCTION OF RUBIACEAE FAMILY:
The Rubiaceae are trees, shrubs, or infrequently herbs comprising about 450 genera and 6,500 species, including some lianous forms.
The leaves are simple and usually entire, and are opposite or sometimes whorled; stipules are present and interpetiolar.
The flowers are nearly always bisexual and actinomorphic, often heterostylous, and usually are in cymose inflorescences.
The fruit is variable, sometimes forming multiples.
The calyx is mostly somewhat reduced and 4-5-lobed or sometimes the lobes are obsolete or rarely one of them greatly expanded and brightly colored.
The sympetalous corolla is mostly 4-5-lobed, occasionally with 3 or up to 10 lobes.
The androecium consists of as many stamens as corolla lobes and is adnate to the corolla tube or epigynous zone, alternate with the lobes.
The gynoecium consists of a single compound pistil of 2 or seldom more carpels, a single style, and a nearly always inferior ovary with the number of locules equaling the number of carpels, each with 1-many axile ovules. An epigynous nectary disk is usually present.
Past work reported on some plants of Mussaendagenus
Mussaendaerythrophylla
Mussaendaerythrophylla (Rubiaceae) is native to western tropical Africa, occasionally seen in gardens and parks as ornamental plant in India and is commonly known as mussenda (telugu), nagavalli (Sanskrit) and red flag bush (English)(33).It is a perennial, evergreen shrub with branched tap root system.
Phytoconstituents reported:
Qualitative phytochemical screening of ethyl acetate extract of M.erythrophyllastem revealed the presence of steroids(β-Sitosterol), triterpenoids ( 3-iso cumaryloxy-cyclopropane-1-oic acid ) and flavonoids ( 5-hydroxy-7, 4’-dimethoxy flavones ). Phytochemical analysis reveal that Mussaendaerythrophylla stem contains phytosterols, triterpenoids, flavonoids, saponins, glycosides and tannins.
Biological activities reported:
Roots are useful for cough, jaundice, when chewed acts as an appetizer.The ethyl acetate and methanolic extracts from roots of M. erythrophylla showed concentration dependent anthelmintic activity against earthworms.
Natural antioxidants such as phenolic acids, flavonoids and tannins possess potent antioxidant activity(30).Sterols like ßsitosterol have been reported for antioxidant activity(31).Terpenoids are also reported to possess antioxidant activity (32).
Mussaendafrondosa(Dhobi tree)
It is found from Indo-China to Malaysia. And also is distributed in Central Nepal, India and Srilanka. It is somewhat smaller and more upright than the above two species, 6 to 9 ft tall, with an equal spread. The foliage is a lighter green, and the terminal flower clusters have orange to yellow, tubular corollas with a single white enlarged calyx lobe. This species is often grown in clumps(04).
Phytoconstituents reported:
The phytochemical screening subjected to detect the presence of some secondary plant metabolites. Ethyl alcohol extract revealed the presence of carbohydrates, steroids, alkaloids, terpenoids, flavanoids, tannins and poly phenols, while aqueous extract showed presence of carbohydrates, alkaloids, flavanoids, tannins and poly phenols.
Biological activities reported:
that alkaloids and flavanoids shows antioxidant property and their effects on human nutrition and health care are considerable(Kumpulainen). Mechanism of action of alkaloids are through inhibition of roxidation(20, 21)
Compounds such as flavanoids are responsible for inhibition of lipid peroxidation(22). Scavenging activity of free radicals of 1,1 diphenyl-1,2-picryl hydrazyl(DPPH) has been widely used to evaluate the antioxidant activity of plants. The DPPH radical scavenging activity increases with increasing concentration. Ethyl alcohol and aqueous extract of MussaendaFrondosaexhibited good reducing power. The juice of the root is used to treat blemishes on the tongue and the sepals are diuretic(05).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Mussaendaraiatensis
Mussaendaraiatensis, a native of Tonga occurs in open places on ridges, in coastal to lowland forests, and is occasionally cultivated for its medicinal properties.
Phytoconstituents reported:
The preliminary phytochemical screening of the methanolic extract of Ant+igononleptopusrevealed that presence of steroids, flavonoids, tannins, alkaloids and glycosides, carotenoids, phenols, coumarins, lignans, essential oil, lipids, monoterpenes, xanthenes and organic acids.
Biological activities reported:
The methanolic extract of aerial parts of Mussaendaraiateensispossesses significant hepatoprotective activity and may prove to be effective for the treatment of liver disorders.
The infusion of the bark is sometimes given to an infant believed to be ill or malnourished(15).
Mussaendamacrophylla
Mussaendamacrophyllais found widely in Central and Eastern Nepal to about 1800 m in moist places inassociation with herbs and other shrubs. It is also found to occur in northern India, Southeastern China and Myanmar(11).
Phytoconstituents reported:
Four new triterpenoid glycosides were isolated from the root bark of Mussaendamacrophylla. Their structures were determined as 3-O-beta-D-glucopyranosyl-28-O-alpha-L-rhamnopyranosyl-16alpha- hydrox y-23-deoxyprotobassic acid (1), 28-O-beta-D-glucopyranosyl-16alpha- hydroxy- 23-deoxy protobassic+++acid (2), 3-O-beta-D-glucopyranosyl-28-O-alpha-L-rhamnopyranosyl-16alpha- hydroxyprotobassic acid (3), and 3-O-[beta-D-glucopyranosyl-(1-->6)]-O-alpha-L-rhamnopyranosyl-(1-->2)-O-beta-D-glucopyranosyl-(1-->2)-O-beta-D-glucopyranosyl-(1-->3)-O- beta-D-glucopyranosyl-cycloarta-22,24-dien-27-oic acid (mussaendoside W, 4). Four known triterpenoids [3-O-acetyloleanolic acid (5), 3-O-acetyldaturadiol (6), rotundic acid (7), and 16alpha-hydroxyprotobassic acid (8)] were also isolated.
Biological activities reported:
Oleanolic acid derivatives isolated fi'om the root bark of M. macrophylla exhibited differential inhibitory activity on the growth of Porphyromonasgingivalis, thegram-negative anaerobic oral bacterium, and most commonly associated with human gum disease 8. 3-O-Acetyloleanolic acid isolated from M macrophylla inhibited the growth of Porphyromonasgingivali.
Mussaendapubescens
Mussaendapubescensis a liana-like shrub, distributed in shady hillside, valley and shrub jungle of East, South andSouthwest China.
Phytoconstituents reported:
Two new triterpenoidsaponins, named mussaendosides G and K, were isolated from aerial parts of Mussaendapubescens by normal and reverse phase chromatography. On the basis of chemical and spectroscopic methods, their structures have been elucidated as heinsiagenin A 3-O-{alpha-L-rhamnopyranosyl(1-->2)-[beta-D-glucopyranosyl(1-->6)]-beta-D-glucopyranosyl(1-->2)}-alpha-L -rhamnopyranosyl(1-->4)-O-beta-D-glucopyranoside and 3 beta,19 alpha-dihydroxyl-olean-12-en-24, 28-dioic acid-24,28-di-O-beta-D-glucopyranoside, respectively.
Biological activities reported:
Mussaendoside F isolated from aerial part of M. pubescens was found to be a muscarinic antagonist (23). Ursolic acid and oleanolic acid lowered blood sugar levels in normal and alloxan-diabetic mice(24). Oleanolic and ursolic acids are known to possess significant anti-inflammatory activities. Both these acids are effective in preventing chemically induced liver injury in laboratory animals. Oleanolic acid has been marketed in China as an oral drug for human liver disorders. Recently these compounds have been noted for their anti-tumour promotion effects(25).
It has been used in Chinese folk medicine as a diuretic, antichloristic and antipyretic agent. The wholeplant of Mussaendapubescens has been used against laryngopharyngitis,
· Acute gastroenteritis
· Dysentery and
· As a contraceptive agent 2
Mussaendaroxburghii
It is distributed in the Eastern and Central Nepal at an altitude of height 200-1200 m in moist shady places ofBhutan, Bangladesh, and Myanmar.
Uses: A paste of the root is applied to the tongue to treat boils (11).
Mussaendaincana
It isnative from India to Malaysia and is much smaller than the above mussaendas, growing to no more than3 ft tall. It has flat-topped flower clusters (corymbs), with bright yellow corollas and a single enlarged calyx lobethat is yellow to cream.
Uses: In the landscape it is most effective in mass plantings.14
· Look for thecultivar ‘White Wings’.
Mussaendaphilippica
It is native to The Philippines, and is known commonly as virgin tree or, less often, tropical dogwood, and forms a shrub or small tree 9 to15 ft tall. and cultivated for its showy habit, colourful scarlet, white or pink sepals and yellow petals (34). It is a bushy shrub growing up to 2.00m and 3.00m for white and pink varieties respectively and both varieties are similar in morphology and ecology, but differ in physiology (35). It is generally planted for its elegance as shrubbery borders or grown as a specimen or focal plant. It flowers luxuriantly formost part of the year and has proved to be the most popular flowering shrub in the warm humid region (36). The medical uses of leaves, flowers and roots of Queen of the Philippines (Mussaendaphilippica) for various ailments have been reported in Ghana, Liberia, Sierra-Leone and China (37,38), but it is mainly used as ornamental plant.Ornamental plants are widely grown for their beauty in form of radiant and showyappearance.
Uses: The sepals of cultivars are active (19).
· Sanshiside methyl ester posssess antiviral property (10) .
· Non glycosidiciridoids like Mussaein are cytotoxic(06).
Mussaendaglabra
It is also found from India to Malaysia and at 2 ft is even shorter than M. incana. It is commonly known as dwarf mussaenda. The tubular corolla is orange to red with an enlarged white calyx lobe(39).
Uses: It is used to best advantage massed in a border.
· This is the most cold tolerant of the cultivated musseandas.
· It is sometimes treated as an annual in the Gulf Coast states.
Mussaenda ‘QueenSirkit’
It was developed by backcrossing the F1 hybrid between M. ‘Aurorae’andM. erythrophylla to M. ‘Aurorae’. It is among the most spectacular of mussaendas, with all five calyx lobes enlarged up to 3½”, in shades of ivory to pale pink. These large flower clusters (panicles) are somewhat fragile; during heavy rain they can become heavy, causing smaller branches to break(14). They also are prone to break off the plant during high winds.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Mussaenda 'dona aurora'
Mussaenda 'dona aurora'(sepals) has been investigated for its hepatoprotective and antioxidant activities. The highest activity was observed in the ethyl acetate fraction. The separation of the ethyl acetate fraction gave two iridoids, sanshiside-D and lamalbide. Sanshiside-D exhibited a hepatoprotective activity greater than silimarin as was evidenced by significant reduction of ALT and AST in the serum enzyme levels.
CONCLUSION
Medicinal plants have played an essential role in the development of human culture. Many of the modern medicines are produced indirectly from medicinal plants, for example aspirin. The study of medicinal plants has progressed is in the discovery of bioactive compounds from new promising drug species and understand plant toxicity and protect human and animals from natural poisons.
The genusMussaenda (rubiaceae) is an important source of medicinal natural products, particularly iridoids, triterpenes and flavonoids, carbohydrates ,poly phenols.
Although a number of chemical components described for the Mussaendagenus are also found in other species, the secondary metabolites produced by this genus, particularly the flavonoids make these plants an important source of potential phytotherapeutic and medicinal agents.
Most of the species reported positive for triterpenoids, steroids, flavonoids, glycosides and only a few number of species reported positive for alkaloids and tannins. Many Mussaenda species were reported to possess anti-oxidant, anti-inflammatory in different models, analgesic, antimicrobial, diuretic, antiphlogistic and antipyretic, acute gastroenteritis and dysentery, anti- fertility activity, antiviral property , antibacterial effect rarely for hepatoprotective activity and wound healing activity.
References
1. BiswanathDinda, SudhanDebnath, SantanuMajumder. 2005, Chemical constituents of Mussaendaincana. Ind. J. Chem.44B (11):2362-2365.
2. Dictionary of Chinese Traditional Medicine, Jiangsu New Medical College (1986) p. 176. Shanghai Science andTechnology Press.
3. Encyclopedia of Fujian Plant Medicines, Fujian Institute of Medicine (1979) Vol. 1, p. 447. Fujian People’s Press.
4. Huxley, A., M. Griffiths, and M. Levy. (eds.).1999. The new Royal Horticultural Society dictionary of gardening,Vol. 3.Groves Dictionaries, Inc., New York. Pp.271 – 272.
5. Jayasinghe, U.L.B, C.P.Jayasooriya, B.M.R Bandara et al. 2002, Antimicrobial activity of some Sri Lankan Rubiaceae and Meliaceae. Fitoterapia. 73(5):424-7.
6. Jing-Qiu Dai, Zhong-Li Liu, Li Yang. 2002, Non-glycosidiciridoids from Cymbariamongolica. Phytochemistry. 59:537–Jun-Ping
7. Xu and Ren-Sheng Xu. 1992, Mussaendosides M and N, New SaponinsFromMussaendaPubescens. J. Nat.Prods.55:1124-1128.
8. Kim, N.C., A.E. Desjardins, C.D.Wu and A.D. Kinghorn. 1999, Activity of triterpenoid glycosides from the root bark ofMussaendamacrophyllaagainst oral pathogens.J. Nat. Prods. 62:1379-1384.
9. Ranarivelo, Y, A.L Skaltsounis, M. Andriantsiferana and F. Tillequin. 1990, Glycosides fromMussaendaarcuataLam. ExPoiret leaves. Ann Pharm Fr. 48(5):273-7.
10. SunitSuksamram, KanjanaWongkrajang, KanyawimKirtikara. 2003, Iridoidglucosides from the sepals of Barlerialupulina. Planta Med 69:877-9.
11. Narayan P Manandarand Sanjay P Manandar. Plants and People of Nepal, Timber press 327: 2002.
12. Weimin Zhao, Genjin Yang, RenshengXu, GuoweiQint. 1996. Three Monoterpenes from MussaendaPubescens.Phytochemistry. 41(6):1553-1555.
13. Weimin Zhao, Jean-Luc Wolfender, Kurt Hostettmann et al. 1997. Triterpenes and triterpenoidsaponins from Mussaendapubescens. Phytochemistry. 45(5):1073-1078.
14. Whistler, W.A.. 2000. Tropical ornamentals: aguide. Timber Press, Portland, Oregon.542 pp
15. WHO, 1998. Medicinal plants in the South Pacific. Ed Michael Doyle. WHO regional publications. Western pack series. Pp125
16. Yaolan Li, Linda S. M. Ooi, Hua Wang, Paul P. H. Butand Vincent E. C. Ooi. 2004, Antiviral activities ofmedicinal herbs traditionally used in southern mainland China. Phytotherapy Research 18 (9): 718 – 722
17. Yoshio Takeda, Hiroshi Nishimura, Hiroyuki Inouye. Two new iridoidglucosides from MussaendaparvifloraandMussaendashikokianaPhytochemistry 1977; 16(9):1401-1404.
18. Vidyalakshmi KS, Charles Dorni AI, Hannah R.Vasanthi. Antimitotic and cytotoxic activity of Mussaendaqueensirikit. J.Pharm.Toxic. 2007; 2 (7):660-5.
19. Kumpulainen JT, SalonenJT(1999). Natural Antioxidants and Anticarcinogens in utrition,Health and Disease,the Royal Society of Chemistry,UK,pp.178-187.
20. Kessler M,UbeaudG,Jung L(2003).Anti and prooxidant activity of rutin and quercetinderivatives.J.Pharm and Pharmacol.55:131-14
21. Cook NC,Samman,S(1996).Flavanoids-chemistry ,metabolism,cardio-protective effects and dietary source.Nutr.Biochem.7:66-76.
22. Das NP,Pereira TA(1990).Effects of Flavanoids on Thermal auto-oxidation of palm oil: ructureactivityrelationship.J.Am.Oil Chem.Soc.67:255- 258.
23. Lakshmi,D.K.M., Girija, A. R., Rao, D.V., Rao, E.V.,Indian J. Pharm. Sczl, 1985,47, 22.
24. Perez, F. R. 1., Perez, G. C., Perez,G. S., Zavala, S. M., Phytomedicine, 1998, 5, 475.
25. Mahmood, U., Shukla,Y. N., Thakur, R. S., Phytochemistry, 1983, 22, 167.
26. XU JP, XU RS, LUO Z, Dong JY. Actachimsinica 1991; 49:621.
27. XU JP, XU RS, LUO Z, Dong JY, HU HM. Journal of Natural Products 1992; 55(8): 1121-1128.
28. Weimin Zhao, Junping XU, Guowei QIN, Rensheng XU. Phytochemistry 1995; 39:191-193.
29. Jefferey B, HorborneGirija AR, Maheshwari Devi H, Lakshmi KM. Phytochemistry 1983; 22:2741-2742.
30. Sanchez-Moreno C. Food ScitechnolInt 2002; 8: 121-137.
31. Cai YZ, Luo Q, Sun M, Corke H. Life Sciences 2004; 74: 2157-2184.
32. Dragland SM, Senoo H, Wake K, Holte K, Blomhoff R. J Nutrition,2003;133:1286-1290.
33. Singh V, Pande PC, and Jain DK. “A text book of botany Angiosperms”. Ist edition. 2000; 156.
34. Rosario, T.L. 1984. The ornament Mussaenda of the Philippines, Inst. Plant Breeding Bul.4.College of Agriculture, UNW. Philippines of LOS Barios, College, Laguina.
35. Sharma, S.C., Sharga, K.N., Sanita, S.1990. Horto-taxonomical studies of some mussaenda. Plantman12(3) 184-187.
36. Bose, T.K. 1981. Knowing a fine flowering shrub. J. of India Horticulture. July – September. P7 and 27.
37. Dalziel, JM. 1957. The useful plants of west Tropical Africa. PP.405
38. Liu,X.J., Liang,C.J., Cai,X., Chao,Q., Chu,Y.H.,Bao, Y.M.,Long, X.H.Wang, G.Q.1986 Acta Acad. Med. Shanghai 13, 273.
39. Huxley, A., M. Griffiths, and M. Levy. (eds.). 1999. The new Royal HorticulturalSociety dictionary of gardening, Vol. 3. Groves Dictionaries, Inc., New York. Pp 271 – 272.
40. Alejandro GD, Razafimandimbison SG, Liede-Schumann S. Polyphyly of Mussaenda inferred from ITS and trnT-F data and ITS implication for generic limits in Mussaendeae (Rubiaceae) American Journal of Botany. 2005;92: 544–557.
41. Silverstein RM, Bassler GC, Morill TC. Spectrometric Identification of Organic Compounds, 4th ed, USA; John Wiley and Sons: 1981.
42. Harborne JB, Baxter H. The Handbook of Natural Flavonoid Vol.2. England:John Wiley & Sons; 1999.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









