 About Authors:
About Authors:
K.S.Khatri
S.K.Patel education and research, Kherva,
Mehasana-384001
kkhatri_pharm@yahoo.com
Abstract:
Interest in transdermal drug delivery systems has increased on several fronts over the past several years because of the inherent advantages over the oral delivery such as the avoidance of gastrointestinal absorption, enzymatic and pH associated deactivation. Stratum corneum forms highly lipophilic membrane and provides the greatest resistance to penetration of drugs. A number of chemical and physical permeation enhancement techniques have been investigated. Chemical enhancers represent the most widely studied approach to transdermal drug permeation enhancement but are associated with toxicity, therefore limiting their clinical application. In recent years the emergence of number of physical techniques has expanded the range of drugs that can be delivered transdermally. Promising new technologies involved in enhancing transdermal permeation are Abrasion, Electroporation, Sonophoresis/Phonophoresis, Pressure waves, Iontophoresis, Laser Radiation,Magnetophoresis, Microneedles, Needleless injection and many more. In the present reviewed tried to discuss alternate techniques of enhancing the transdermal transport.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1465
INTRODUCTION
The transdermal route has been recognized as one of the highly potential routes of systemic drug delivery and provides the advantage of avoidance of the first-pass effect, ease of use and withdrawal (in case of side effects), and better patient compliance. However, the major limitation of this route is the difficulty of permeation of drug through the skin. Studies have been carried out to find safe and suitable chemical permeation enhancers to promote the percutaneous absorption of a number of drugs.
Advantages of Transdermal Drug Delivery:
* Transdermal delivery avoids the stomach environment where the drug can be degraded and rendered ineffective or where it can cause unpleasant gastrointestinal symptoms for the patient.
* Transdermal delivery avoids the first pass effect where active drug molecules can beconverted to inactive molecules or even tomolecules responsible for side effects.
* Transdermal drug delivery provides steady plasma levels. When a patch is applied thatlasts for 24 hours, or even 7 days, oncesteady state is reached the plasma levelsremain constant because the rate of drug delivered from the patch is constant. When adrug is given four times a day, or even once a day, the drug levels rise afteradministration and then gradually fall until the next administration producing peaks andtroughs throughout the course of therapy.
[adsense:468x15:2204050025]
Other advantages to delivering drugs through the skin include the fact that:
v Transdermal drug delivery systems, especially simple patches, are easy to use and noninvasive and patients like noninvasive therapies.
v Because they are easy to use, patches can increase compliance and reduce medical costs.
v If a transdermal delivery system is used in place of a needle, then medical waste can also be decreased, again, decreasing healthcare costs.
Disadvantages of Transdermal Drug Delivery:
v One of the greatest disadvantages to transdermal drug delivery is the possibility that a local irritation will develop at the site of application. Erythema, itching, and local edema can be caused by the drug, the adhesive, or other excipients in the patch formulation.
v Another significant disadvantage of transdermal drug delivery is that the skin's low permeability limits the number of drugs that can be delivered in this manner. Because the skin serves protective functions, it inhibits compounds from crossing it. Many drugs with a hydrophilic structure permeate the skin too slowly to be of therapeutic benefit. Drugs with a lipophillic character, however, are better suited for transdermal delivery. Many of the recent developments in transdermal drug delivery target the more hydrophilic compounds that were previously undeliverable via this method.
v Damage to a transdermal patch, particularly a membrane or reservoir patch, can result in poor control over the release rate. The release rate from a damaged patch would more likely be controlled by the skin than the patch, resulting in a higher, perhaps toxic, rate of drug delivery. Patients should be advised to discard a patch if the outer packaging or the patch itself appears damaged or altered in any way.
v Currently, only small quantities of drug can be delivered through the stratum corneum. Therefore, drugs that are given transdermally must be relatively potent so that they can be effective at low doses.
1st GENERATION Transdermal Drug Delivery Systems:
The original patch design is a liquid reservoir system where the patch consists of a backing material that is both protective and adhesive, a liquid drug reservoir, a release membrane.
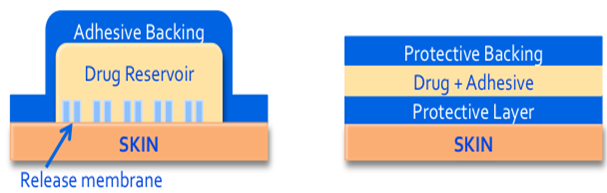
A more recent design is the adhesive matrix system where the adhesive and the drug are combined in the same layer leaving only three layers to the patch; the backing layer, the drug and adhesive layer, and the protective layer that would be removed before applying the patch to the skin. Most currently available patches, except those previously mentioned are the adhesive matrix design.
2ND GENERATION Transdermal Drug Delivery Systems:
2nd Generation TDDS attempt to enhance the delivery of organic molecules through the stratum corneum by disrupting its barrier function and/or by providing some sort of driving force for the movement of molecules through the epidermis. This disruption should be reversible and avoid injury to the skin. However, it can be difficult to disrupt the barrier without causing damage or irritation, especially when using chemical enhancers. In addition, these 2nd generation enhancement techniques are limited to small, lipophilic molecules and still have little effect on larger or hydrophilic molecules. 2nd generation enhancement methods include chemical penetration enhancers, gentle heating.
Chemical Penetration Enhancers:
DMSO is a polar, aprotic solvent that can disrupt the protein structure of the keratinocytes. Because it acts on the keratinocytes, DMSO will increase movement of drug molecules through the keratinocytes and enhance the transcellular route of delivery.
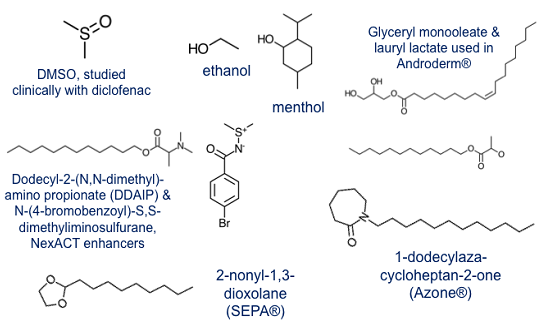
Small solvent molecules like ethanol and menthol will increase the solubility of drug molecules in the lipid bilayer and thereby enhance the intercellular route of drug movement.
Molecules whose structures mimic that of phospholipids, those with a small, polar head and a long, hydrocarbon tail, will insert into the lipid bilayer and increase the fluidity within that layer. If the bilayer is more fluid, it will be easier for drug molecules to move through it, also enhancing intercellular movement. Examples include glyceryl monooleate and lauryl lactate which are currently used in Androderm®, as well as Azone TS ®, and the two NexACT® enhancers shown.
Heat as a penetration enhancer:
Another form of penetration enhancement is the use of heat to increase the permeability of the skin. One safe use of heat as a penetration enhancer is the Controlled Heat-Assisted Drug Delivery, CHADD, system. In a CHADD system, a mix of proprietary powders reacts with the air to generate heat that then warms the skin and increases the delivery of the drug. This heat device can be placed on top of an existing patch or other medication or it can be manufactured in combination with a drug of choice.
The most well known of the CHADD systems is the lidocaine/tetracaine patch system which goes by the brand name Synera®. This system is made by ZARS Pharma and is advertised as the “precedural” treatment before a needle stick to reduce the pain of the procedure. The gentle heat is combined with a lidocaine/tetracaine mix that causes effective analgesia within 20 minutes. If a needle-stick procedure can wait 20 minutes, this can be a great way to make a needle stick easier for a child.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3rd GENERATION Transdermal Drug Delivery Systems:
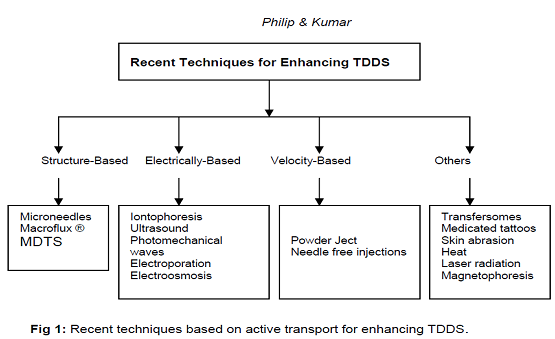
Structure-Based Enhancement Techniques:
Microneedles for Transdermal Drug Delivery:"
Drugs with poor oral bioavailability usually are administered by hypodermic injection, which causes pain, poor patient compliance, the need for trained personnel, and risk of infectious disease transmission. Transdermal (TD) delivery provides an excellent alternative, but the barrier of skin’s outer stratum corneum (SC) prevents delivery of most drugs. Micrometer-scale microneedles (MNs) have been used to pierce animal and human cadaver skin and thereby enable TD delivery of small molecules, proteins, DNA, and vaccines for systemic action.
Advantages:
• Delivers significant benefits compared to injection:
- Improved delivery efficiency of some drugs and vaccines
- Quicker onset of action for some drugs
- Potential to reduce drug costs by using less drug to achieve similar efficacy
• Potential to generate unique pharmacokinetic profile, difficult for competitive products to match
• Helps improve compliance compared to injection, based on the minimal discomfort associated with application and ease of self-administration
• Potential to reduce or eliminate side effects associated with other dosage forms
• Manufacturing expertise to meet your needs from clinical supplies to commercial scale.
Solid Microneedle System (sMTS):
With up to 0.3 mg capacity, sMTS provides delivery of highly potent proteins or vaccines:
• 375-1300 solid microneedles per cm2, 150-700μm tall, each coated with drug or vaccine
• 3M precision coating technology used to uniformly apply the drug or vaccine to tips of microstructures
• Upon application, microneedles penetrate stratum corneum and remain in the skin
• Wear time is short, ranging from 30 seconds to 10 minutes
• May enhance stability, allowing room temperature storage
• Single and multiple use applicators available
• For vaccines, able to induce higher immune response with potential to reduce number of required doses.
Hollow Microneedle System (hMTS):
With up to 2 mL capacity, hMTS is designed for efficient delivery of liquid formulations:
• 18 hollow microneedles per cm2, approximately 950μm tall
• Upon application, microneedles penetrate the skin to allow for fluid flow from the device into the skin
• Wear time ranges from 2 to 20 minutes
• Infusion time dependent on formulation (e.g. viscosity and volume)
• Intradermal infusion at higher volumes than most injections
• Provides potential for improved PK relative to subcutaneous injection for a variety of drugs, including monoclonal antibodies
• Potential to achieve drug levels similar to subcutaneous injection for smaller compounds
• Demonstrated infusion of 1.0 mL in less than one minute
Dissolving Microneedles for Transdermal Drug Delivery:
To address limitations of oral delivery and hypodermic injection, arrays of micron-scale needles have been developed to painlessly pierce skin’s outer barrier of stratum corneum with the goal to deliver drugs with the efficacy of a needle and the convenience of a transdermal patch. This approach has demonstrated increased transdermal delivery of small-molecule drugs, proteins, DNA, and vaccines. One approach involves pretreatment of skin with microneedles, followed by application of a transdermal patch for extended drug delivery through the permeabilized skin. Another approach involves coating or encapsulating drug onto or within microneedles. Upon dissolution of the coating or the needle itself, the drug cargo is released within the skin as a bolus or possible controlled-release delivery.
Micro fabrication tools have been leveraged to make microneedles using methods suitable for low-cost, high-volume manufacturing, which is critical to impacting medicine as a disposable device. Microneedles suitable for piercing skin to increase skin permeability or for carrying drug into the skin as a coating have been fabricated from silicon and metal. Microneedles that encapsulate drug and subsequently dissolve or degrade in the skin have been fabricated from polymers, such as slow-degrading polylactic-co-glycolic acid and rapidly-dissolving sugar. To address growing needs of protein delivery, we propose that a microneedle device should (1) encapsulate drug within a biocompatible and mechanically robust material using processes that do not damage protein integrity, (2) enable controlled delivery as a bolus or sustained release, and (3) utilize a device suitable for self-administration without medical training that leaves behind no sharp, biohazardous waste. This study presents microneedles designed to have these attributes using polysaccharide biomaterials; a gentle microneedle molding technique; and study of mechanical, stability, and delivery properties using model proteins and cadaver skin.
Significance to drug delivery:
Dissolving microneedles designed in this study may enable (1) bolus and sustained delivery of drugs into the skin, (2) self-administration of drugs that would otherwise require a hypodermic needle and (3) elimination of dangers associated with improper needle disposal and intentional re-use, especially in the developing world. microneedles that dissolve or degrade in the skin have either melted polymer into a mold at high temperature, which can damage sensitive biomolecules, or have hand assembled individual needles of millimeter dimensions, which is a poorly controlled and non-manufacturable process. Another feature of dissolving microneedles is that the backing membrane to which microneedles are attached has been used, for the first time, as a drug reservoir for sustained drug delivery over many days. Previous approaches have encapsulated drug only within microneedle shafts or, alternatively, coated drug onto the surface of microneedle shaft. These approaches have been limited to small (e.g., microgram) drug doses due to the inherently small volume and surface area of microneedles. Use of the backing membrane as a drug reservoir may increase total doses to milligrams. This two-step process may be cumbersome for patients and susceptible to mistakes. Researcher mimicked this scenario, but accomplished it as a one-step process by using dissolving microneedles, which did not need to be removed after piercing the skin and were integrated onto the surface of a backing layer that served functionally as a transdermal patch. By controlling microneedle patch design, release kinetics was controlled over times ranging from minutes to days. Further design and formulation changes should be able to achieve a variety of different bolus and sustained release profiles.
Release from the backing layer reservoir probably occurs by a set of interacting phenomena. First, microneedles insert the skin and dissolve, which creates channels for drug transport into skin and for interstitial fluid transport out of skin. Although drug may diffuse through a dry backing layer, drug diffusion should be enhanced by hydrating the backing layer with interstitial fluid from skin. An example of this swelling is shown in Fig, where a dissolving microneedle patch shows extensive swelling after 15 h on the skin (Fig. 9a). As a negative control, a patch backing layer fabricated without microneedles was also placed on skin, but did not swell after placement for the same time (Fig 9b).
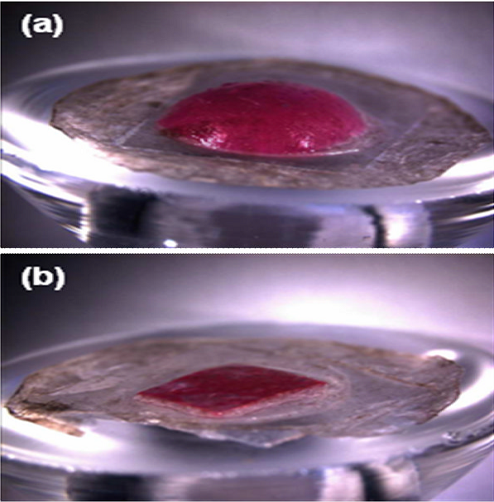
Swelling of dissolving microneedle patch backing layer after insertion into skin using a Franz cell. (a) A patch of CMC pyramidal microneedles containing sulforhodamine inserted into skin for 15 h shows extensive swelling of the backing layer. (b) A backing layer of CMC that contains no microneedles (negative control) placed on the surface of skin for 15 h shows little swelling. Human epidermis was used. Imaging was by bright field microscopy.
This suggests that the patch backing layer swelled by imbibing interstitial fluid from skin through channels created by microneedles. This observation is not only relevant to understanding drug delivery mechanisms, but also suggests uses to extract interstitial fluid for diagnostic applications, such as measuring glucose concentration in diabetics or monitoring industrial toxins in at-risk populations. We found that a model enzyme, lysozyme, encapsulated in CMC microneedles was not substantially degraded after two months storage at room temperature. This stability may be explained by the limited molecular mobility in the solid state that is known to enhance protein stability. Additional studies are needed to determine if other proteins are similarly stable. All of these findings are based on observations in vitro. Additional studies are needed to assess performance in vivo using animals and, ultimately, humans. In addition, even though microneedles were fabricated using materials found in other FDA-approved formulations, dermatotoxicity and irritation have not been fully evaluated and needs to be addressed. Finally, practical applications require robust and cost-effective manufacturing, which needs further study and development.
Coated microneedles for transdermal delivery:
Biopharmaceuticals, such as peptides, proteins and future uses of DNA and RNA, represent a rapidly growing segment of pharmaceutical therapies. These biotechnology drugs are currently delivered almost exclusively by the parenteral route. The oral route is generally not available, due to poor absorption, drug degradation and low bioavailability. This is problematic, because parenteral administration with hypodermic needles requires expertise for delivery, can lead to transmission of blood borne pathogens due to accidental needle sticks or intentional needle reuse, and causes pain, which results in reduced patient compliance due to needle phobia.
Given these problems, a breadth of research activity has focused on replacing hypodermic needles with alternate drug delivery methods. Transdermal drug delivery is an especially attractive alternative, because it is usually easy to use, safe, and painless. However, the tough barrier posed by the skin’s outer layer of stratum corneum has limited the applicability of this method to drugs that are hydrophobic, low molecular weight, and potent. Micron-scale needles assembled on a transdermal patch have been proposed as a hybrid between hypodermic needles and transdermal patches to overcome the individual limitations of both injections and patches. Microneedles have been shown to be painless in human subjects relative to hypodermic needles. Unlike transdermal patches, microneedles have been successfully used to deliver a variety of large and hydrophilic compounds into the skin, including proteins and DNA. In vitro skin permeability enhancement of two to four orders of magnitude was observed for small molecules like calcein and large compounds like proteins and nanoparticles. In vivo delivery has been shown for peptides, such as insulin and desmopressin; genetic material, including plasmid DNA and oligonucleotides; and vaccines directed against hepatitis B, anthrax and Japanese encephalitis. Microneedles can be fabricated for these applications by adapting the tools of the microelectronics industry for inexpensive, mass production. Currently, four different modes of microneedle-based drug delivery have been investigated. These modes are:(1) piercing an array of solid microneedles into the skin followed by application of a drug patch at the treated site; coating drug onto microneedles and inserting them into the skin for subsequent dissolution of the coated drug within the skin; encapsulating drug within biodegradable, polymeric microneedles followed by insertion into skin for controlled drug release; and injecting drug through hollow microneedles. Among these approaches, coated microneedles are attractive for rapid bolus delivery of high molecular weight molecules into the skin, and can be implemented as a simple ‘Band-Aid’- like system for self-administration. Further, storing drugs in a solid phase as a coating on microneedles may enhance their long-term stability, even at room temperature. Consistent with this expectation, desmopressin coated onto microneedles maintained 98% integrity after 6 months storage under nitrogen at room temperature. Coated microneedles are also especially attractive for vaccine delivery to the skin, because antigens can be released in the skin to target epidermal Langerhans cells and dermal dendritic cells for a more potent immune response. As a demonstration of this, a strong immune response in guinea pigs was shown against ovalbumin, a model antigen delivered from coated microneedles. Despite the attractiveness of coated microneedles, a detailed investigation of the coating process has not been published. In this study, we therefore wanted to develop a microneedle coating process that elucidated issues involved in coating microneedles and to identify the breadth of applicability of coated microneedles by determining the different kinds of molecules and particles that can be coated and delivered into the skin. This study was guided by identifying essential characteristics needed for the microneedle coating process to achieve precise dose control, safety, and the ability to coat sensitive biological molecules. The coating process should: (1) make a uniform coating as opposed to a patchy coating to provide reproducibility and dosage control, (2) limit deposition only onto microneedles and not on the base substrate for tight dosage control and minimizing drug loss during coating, (3) avoid high temperatures to maintain drug integrity, (4) use aqueous coating solution to prevent denaturing of proteins and other biological molecules, (5) achieve high drug loading per microneedle to maximize drug dosage, (6) provide good adhesion of the coating to the microneedle to prevent wiping off on the skin during insertion and (7) have rapid, or otherwise controlled, dissolution kinetics in the skin for bolus, or sustained, release. Among the various coating processes, such as dip coating, roll coating and spray coating, dip coating is particularly appealing for coating microneedles because of its simplicity and its ability to coat complex shapes. A dip-coating process typically involves dipping and withdrawing an object from a coating solution, after which a continuous liquid film adheres and dries on the object’s surface, leaving behind a uniform coating. However, dip coating has been developed to coat macroscopic objects mostly by submerging them completely within the coating solution. Because surface tension becomes dominant on the micron scale, conventional dip-coating methods have difficulty controlling coating of specified sections of micron-dimensioned structures, especially when those structures are closely spaced. This study sought to addresses these limitations by developing a micron-scale, dip-coating process to coat microneedles with uniform and spatially controlled coatings using methods applicable to a breadth of drugs and biopharmaceuticals.
Macroflux®:
Macroflux® technology is another novel transdermal drug delivery system that ALZA Corporation has developed to deliver biopharmaceutical drugs in a controlled reproducible manner that optimizes bioavailability and efficacy without significant discomfort for the patient. The system incorporates a titanium microprojection array that creates superficial pathway through the skin barrier layer to allow transportation of therapeutic proteins and vaccines or access to the interstitial fluids for sampling. Macroflux® has an area of up to 8cm2 and contains as many as 300 microprojection per cm2 with individual micro projection length being < 200μm. The maximal adhesive patch size is 10 cm2. A coating process is used to apply drug to the tip of each microprojection in the array. When the patch is applied to the skin, the drug-coated microprojections penetrate through the skin barrier layer into the epidermis. The microcapillaries for systemic distribution absorb the drug.
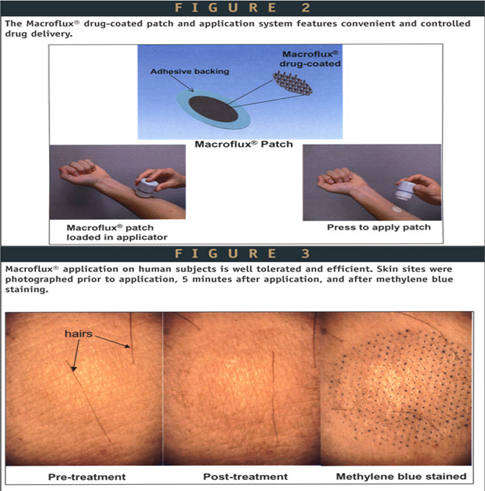
The rate of absorption is promoted by the high local drug concentration around the microprojections and the large surface area provided by the patch array. Three types of Macroflux ® have been designed and tested in preclinical studies. They include,
• Dry-Coated Macroflux ® system for short duration administration that consists of a drug coated microprojection array adhered to a flexible polymeric adhesive backing.
• D-TRANS® Macroflux® system for short duration administration that consist of a microprojection array coupled with a drug reservoir.
• E-TRANS® Macroflux ® system for pulsatile or on demand delivery that include a microprojection array coupled with an electrotransport system.18 Therapeutic peptides, proteins and vaccines such as desmopressin, human growth hormone (HGH), TH 9507 (a human growth hormone releasing factor analog), ovalbumin(45000 Da protein) are in the developmental stage for transdermal delivery by Macroflux®.
Metered-Dose Transdermal Spray (MDTS):
Metered-dose transdermal spray (MDTSTM), originally developed at the Victorian College of Pharmacy [Monash University (Parkville Campus), Parkville, Victoria, Australia] and currently being commercialized by Acrux Limited [Melbourne, Victoria, Australia] has the potential to expand the growth of TDD systems by broadening patient acceptance and pharmaceutical applications for enhanced TDD. MDTS relies on the combination of a newly identified GRAS (generally recognized as safe) chemical penetration enhancer (AcrossTM) and volatile: nonvolatile vehicle. This MDTS can be classified, as an enhanced, passive TDD system.
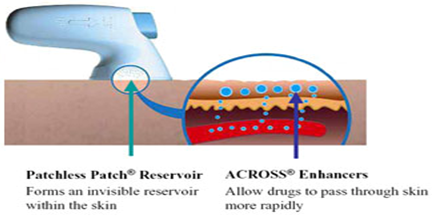
It is a topical solution made up of a volatile cum nonvolatile vehicle containing the drug dissolved as a single-phase solution. A finite metered - dose application of the formulation to intact skin results in subsequent evaporation of the volatile component of the vehicle, leaving the remaining nonvolatile penetration enhancer and drug to rapidly partition into the stratum corneum during the first minute after application, resulting in a stratum corneum reservoir of drug and enhancer. Following a once daily application of the MDTS, a sustained and enhanced penetration of the drug across the skin can be achieved from the stratum corneum reservoir. Different types of penetration enhancers, such as ethanol and azone, are commonly used. Clinical experiences with estradiol-MDTS to post-menopausal women have shown increased higher plasma level of estradiol than the baseline value measured by radioimmunoassay. The MDTS has the following potential advantages:
1. Enhanced passive tdds with little or no skin irritation primarily as a result of its nonocclusive nature.
2. Improved cosmetic acceptability
3. Dose flexibility
4. Simplicity of manufacture.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Iontophoresis:
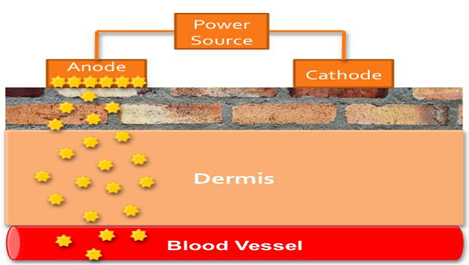
Iontophoresis may be defined a the facilitation of ionizable drug permeation across the skin by an applied electrical potential, the driving force of which may be simply visualized as electrostatic repulsion.22 A typical iontophoresis device consists of a battery, microprocessor controller, drug reservoir and electrodes. Iontophoresis is the process of using small amounts of electrical current to move drugs across the skin and can also be used to enhance penetration of drug molecules through the stratum corneum.
In a Galvanic, or Voltaic, cell, the cathode is the electrode that attracts positively charged ions from the solution, thus the word “cation” to describe a positively charged ion such as Na+. Likewise, the anode is the electrode that attracts negatively charged ions from the solution, thus the word “anion” to describe a negatively charged ion such as Cl-. If an anode attracts negatively charged particles, then it will repel positively charged ones.
In an Iontophoresis system the anode will repel positively charged drug molecules. Because most drugs are formulated as a salt, for instance fentanyl hydrochloride (Figure 8), the drug molecule becomes protonated and takes on a positive charge to the negative charge of the chloride anion. This means that the drug will be repelled away from the anode and through the stratum corneum toward the dermis.
Several studies have addressed the application of iontophoresis to the delivery of low molecular weight solutes (< 500 Da). For delivery of macromolecules, proteins and peptides such as calcitonin, corticotrophin-releasing hormone, δ- sleep- inducing peptide, dextrin sulphate, inulin, insulin, gonadotropin releasing hormone, growth hormone releasing factor, neutral thyrotrophinreleasing hormone, parathyroid hormone and vasopressin iontophoresis may also be utilized. To date, clinical studies have been limited to smaller molecules such as lidocaine, ketorolac dexamethasone, etofenamate, naproxen, vincristine, cortisone and fentanyl.
Ultrasound:
Still is another 3rd generation technology that can be used to increase delivery of drugs across the skin is the use of ultrasound waves (Prausnitz 2008). Physical therapists and athletic trainers have used ultrasound for many years to deliver small molecular weight molecules such as dexamethasone, ketoprofen, or lidocaine to their patients. Using ultrasound to deliver drugs across the skin is also referred to as phonophoresis or sonophoresis. This is the same type of ultrasound technology that is used in lithotripsy to break up kidney stones and gallstones.
Mechanism:
The mechanism of improved transdermal transport by ultrasound has been studied for the past 20 years. In spite of the large number of studies that have been published, the mechanism is still not well understood or characterized. A possible mechanism of improved percutaneous transport by ultrasound suggested by several groups is that ultrasound might interact with the structural lipids locate in the intercellular channels of the stratum corneum. This is similar to the postulated effects of some chemical transdermal enhancers that act by disordering lipids. Tachibana and Simonin postulated that the energy of ultrasonic vibration enhanced transdermal permeability through the transfollicular and transepidermal routes, suggesting that microscopic bubbles (cavitation) produced at the surface of the skin by ultrasonic vibration might generate a rapid liquid flow, thereby increasing skin permeability. Mitragotri et al. evaluated the role played by various ultrasound-related phenomena, including cavitations, thermal effects, generation of convective velocities and mechanical effects. The authors hypothesized that transdermal transport during low-frequency ultrasound application occurs across the keratinocytes rather than the hair follicles. They suggested that cavitation causes disorder of the stratum corneum lipids, resulting in significant water penetration into the disordered lipid region. This might cause the formation of aqueous channels through the intercellular lipids of the stratum corneum through which permeates could move. Tang et al. studied the relative roles of enhanced diffusion due to ultrasound-induced skin alteration and enhancement due to ultrasound forced convection. The findings (theoretical and experimental) suggest that, for low-frequency ultrasound, the relative contribution depends on the in vitro skin model studied. Specifically, convection plays an important role when heat-stripped stratum corneum is exposed to ultrasound, whereas its effect is minimal when full-thickness skin is utilized. In addition, the effective pore radius of the skin estimated using heat-stripped stratum corneum during
ultrasound exposure is much larger than that within full thickness skin. All recent studies indicate that cavitation plays an important role in the enhancing mechanism. Several attempts have been made to establish a suitable mathematical model that will describe the enhancement phenomenon and predict the enhancement ratio for different drugs in various conditions
Future trends:
Vaccination In recent years, the potential for exploiting the skin for purposes of vaccination has received a great deal of attention. Transcutaneous immunization provides access to the immune system of the skin, which is dominated by densely distributed and potent antigen-presenting cells (Langerhans cells). Langerhans cells have been shown to play essential roles in the induction of T-cell-mediated immune reactions against a wide variety of antigens. In order for this technique to be practical, the vaccine, which is generally a large molecule or complex, has to penetrate the stratum corneum barrier. Normally, skin is not permeable under these conditions. Ultrasound can be used to enhance skin permeability to both the adjuvant and the vaccine, and hence to facilitate their delivery to the target cells.
Gene therapy:
Another future application for ultrasound as a topical enhancer, which seems to show promise, lies in the field of topical gene therapy. Gene therapy is a technique for correcting defective genes that are responsible for disease development, most commonly by replacing an ‘abnormal’ disease-causing gene with the ‘normal’ gene. A carrier molecule (vector) is usually used to deliver the therapeutic gene to the target cell. Topical delivery of the vector–gene complex can be used for target cells within the skin, as well as for the systemic circulation. The identification of genes responsible for almost 100 diseases affecting the skin has raised the option of using cutaneous gene therapy as a therapeutic method. The most obvious candidate diseases for cutaneous gene therapy are the severe forms of particular genodermatoses (monogenic skin disorders), such as epidermolysis bullosa and ichthyosis.
Electroporation:
Electroporation is another recent technique which involves the use of large transmembrane voltages caused by electric pulses (10 μs–100 ms) which create reversible pores in the membrane. Electroporation has been widely used for the last fifteen years in cell biology for different purposes: gene transfer in mammalian cells, and introducing RNA, proteins, dyes, nucleotide and antibodies into cells, lately, it has been explored as a potential transdermal drug delivery technique to compromise the obstacle function of the stratum corneum. The pulse voltage, duration, number and rate are the parameters which help to synchronize the drug delivery through electroporation; the varying effects of these parameters on transdermal transport have been extensively investigated by researchers.
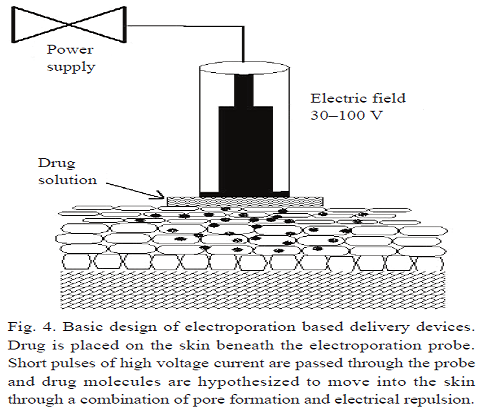
The investigators observed diminished resistance of the skin by three orders of magnitude during electroporation within microseconds. The flux of active substance also reported to increase upto 10–104 fold for neutral and highly charge molecules Attempts were also made to deliver the macro size molecules by Cheng et al. One of the most important developments of electroporation is delivery of physostigmine for organophosphate poisoning. Another remarkable application of electroporation is electro chemotherapy, to increase the bioavailability of cytotoxic drug into tumor cells by applying high voltage pulses. Recently Rastogi et al investigated feasibility of transdermal electroporation for insulin-loaded nanocarriers. It was observed that electroporation of nanoparticles resulted in fourfold enhancement in insulin deposition in rat skin. Finally it was concluded that electroporation of polymeric nanosystems can be successfully used as alternative to injectable administration for the delivery of insulin loaded nanocarriers.
Although electroporation has been shown to be more effective than iontophoresis for several molecules in vitro and produce significant enhancement in skin permeation. Significant increase in drug delivery of cyclosporine A (CSA) has been observed with electroporation than both passive and iontophoresis. Electroporation combined with ethanol was used as the penetration enhancement strategy for the delivery of CSA in the skin for the treatment of psoriasis. It was suggested that multiple pulse electroporation was more effective than single pulse mode in the delivery of CSA to the skin. Electroporation was also claimed to be a safe technique as animal studies indicated that electroporation did not damage the skin, it is supported by evidence that electroporation delivery of chemotherapy was well tolerated by human subjects. Electroporation not only constraints its application to drugs but is also used to deliver macromolecules, though it is still not feasible commercially as side effects like muscle contractions and muscle fatigue.
Electro-Osmosis:
Electroosmotic flow (or electro-osmotic flow, often abbreviated EOF; synonymous with electroosmosis or electroendosmosis) is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or any other fluid conduit. Because electroosmotic velocities are independent of conduit size, as long as the double layer is much smaller than the characteristic length scale of the channel, electroosmotic flow is most significant when in small channels. Electroosmotic flow is an essential component in chemical separation techniques, notably capillary electrophoresis. Electroosmotic flow can occur in natural unfiltered water, as well as buffered solutions.If a charged porous membrane is subjected to a voltage difference, a bulk fluid or volume flow, called electro osmosis occurs without concentration gradients, suggesting that this flow is not diffusion. This bulk fluid flow by electro osmosis was found to be of the order of micro liters per hour per square centimeter of hairless mouse skin. The electro – osmotic flow occurs from anode to cathode, thus enhancing the flux of positively charged (cationic) drugs and making it possible to deliver neutral drugs.
Velocity Based Enhancement Techniques:
Needle-Free Injections:
The highest value, least developed and most technically challenging group of needle-free technologies is prefilled, disposable injectors. The development of such technologies is primarily driven by the demand for a convenient,non-invasive alternative to the conventional needle and syringe injection. The earliest needle free injectors became available as early as 1866, when the French company H.Galante manufactured an “Apparatus for aqua puncture”.18 some of the needle free injectors under development are:
(a)-Intraject®: One of the prefilled disposable injectors, intraject, under development, is designed to use the nitrogen propelled device which has a blank drug capsule. The patient snaps off the tip, tears off the safety end and plenus the nozzle against the skin pressurized gas, and then pushes the liquid formulation through a narrow orifice into the skin.
(b)-Implaject®: Implaject first pushes a tiny, potential “Pioneer tip” thorough the skin ahead of the drug. The tip pierces the tissue, creating a channel through which the therapeutic agent follows immediately.
(c)-Jet Syringe®: The jet syringe, which can deliver up to 0.5 ml; can be configured with an adjustable dose fillable ampoule or proprietary prefilled glass ampoule for fixed dose applications. It is suitable for short-term infrequent injection therapies.
(d)-Iject®: The design of Iject is based on Biojector 2000. It is a light weight, hand-held liquid NFI [Needle-free injectors]. It can deliver 0.1 to 1.0 ml subcutaneously and intramuscularly.
(e)-Mini-ject®: The Mini–ject system utilizes a glass drug cartridge to accommodate for longterm drug storage and stability; a polycarbonate syringe, to accommodate for a wide range of pressure profiles; and a proprietary multiphase energy system that can deliver a specific pressure profile to ensure that the entire drug is delivered comfortably. It can target specific tissue layers including the dermal, subcutaneous and intramuscular layers.
(f)-Crossjet®: It comprises three modules. The gas generator contains the chemical energy source and is triggered by the impact of a syringe, the drug container and the third module, nozzle, of polycarbonate with one or more orifices depending on the quantity of the formulation. The outer layers of the skin using a suitable energy source, usually a compact gas source, is used to propel a pre-measured quantity of liquid medicine through the skin and into the underlying subcutaneous tissue, without the use of a needle. The needle-free devices have been developed for the delivery of drugs such as insulin, sumatriptan and human growth hormone.
Powderject Device:
The core technology involves the high velocity injection of particle formulated drugs and vaccines into any physically accessible tissue. These may be for therapy or prevention of disease and may be small molecules, peptides, proteins and genes. The Powderject system involves the propulsion of solid drug particles into the skin by means of high-speed gas flow. This needle-free method is painless and causes no bleeding and damage to the skin. The use of compressed gas to force solid drug particle through a convergent divergent nozzle was reported by Bellhouse et al. using compressed helium. Drug particle velocities of up to 800 m/s were obtained at the nozzle exit. Adjusting the momentum density of the particles within the gas flow optimizes the depth of penetration of the drug particles.
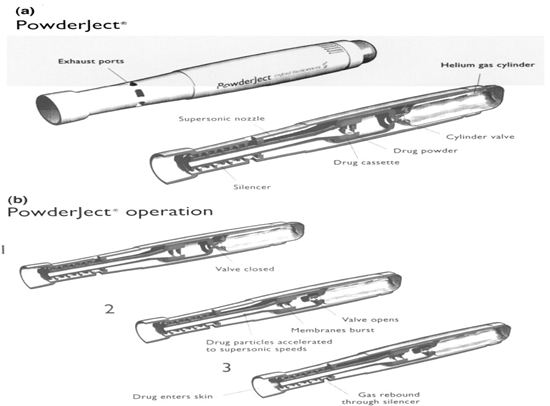
Particle velocity is controlled within the device by three parameters namely nozzle geometry, membrane burst strength and gas pressure Powderject system consists of a gas canister that allows helium gas at high pressure to enter a chamber at the end of which drug cassette containing powdered drug between two polycarbonate membranes. At the release, virtually instantaneous rupture of both membranes causes the gas to expand rapidly, forming a strong shock wave that travels down the nozzle at speed of 600–900 m/s. Powderject device has been reported to successfully deliver testosterone, lidocaine hydrochloride, and macromolecules such as calcitonin and insulin.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Other Enhancement Techniques:
Transfersomes:
To date, the most promising transdermal drug carrier is the recently developed and patented Transfersome® which penetrates the skin barrier along the transcutaneous moisture gradient. This leads the carriers through the “virtual “pores between the cells in the organ without affecting its biological and general barrier properties. Lipid – based suspensions, such as liposomes and niosomes, have been proposed as low-risk drug carriers. Transfersome carriers can create a highly concentrated drug depot in the systemic circulation. Liposomes are microscopic bilayer vesicles, which are usually made of phospholipids (mainly phosphatidylcholine) and cholesterol, contain both hydrophilic and lipophilic portions and can serve as carriers for polar and non polar drugs. Niosomes have a similar morphology, but are made of nonionic surfactants, typically alkyl polyoxyethylene ethers, mixed with cholestrol.Transfersomes contain at least one component that controllably destabilizes the lipid bilayers and thus makes the vesicles very deformable. Additives useful for this purpose are bile salts, polysorbates, glycolipids, alkyl or acyl – polyethoxylenes etc. Transfersome carriers loaded with various agents of different molecular size and lipophilicity (lidocaine, tetracaine, cyclosporine, diclofenac, tamoxifen, etc.) have been shown to cross the skin barrier. In addition, polypeptides such as calcitonin, insulin, α- and γ- interferon, and, Cu – Zn super oxide dismutase, serum albumin, and dextrose have been successfully delivered across the skin with transfersome carriers.
Medicated Tattoos:
Med-Tats is a novel means of delivering compounds transdermally and is produced by Lipper–Man Ltd [Morristown, N.J.]. Medicated Tattoo (Med-Tat) is a modification of temporary tattoo which contains an active drug substance for trandermal delivery. Med–Tats are applied to clean, dry skin in the same manner as traditional temporary tattoos and, according to Lipper – Man Ltd, are not unsightly but rather are attractive and fun to wear. There is no predetermined duration of therapy for Med–Tats; instead, the manufacturer provides a color chart that can be compared to the color of the patient’s tattoo to determine when the tattoo should be removed. This visual comparison, which relies on the dyes incorporated into the patch, introduces a significant amount of interpatient variability. Drugs and other compounds used in Med-Tats prototypes include acetaminophen and vitamin C. The main advantage of medicated tattoos is the delivery of drugs to children who cannot tolerate more traditional dosage forms.
Skin Abrasion:
The abrasion technique involves the direct removal or disruption of the upper layers of the skin to facilitate the permeation of topically applied medicaments. Some of these devices are based on techniques employed by dermatologists for superficial skin resurfacing (e.g., microdermabrasion) which are used in the treatment of acne, scars, hyperpigmentation and other skin blemishes. Microscissuining is a process which creates microchannel in the skin by eroding the impermeable outer layers with sharp microscopic metal granules. Carlisle Scientific [Carlisle, MA] is currently in the process of developing a pen – like handheld device called the microscissioner. In addition, Med Pharm Ltd. [Charlbury, United Kingdom] had recently developed a novel dermal abrasion device (D3S) for the delivery of difficult to formulate therapeutics ranging from hydrophilic low molecular weight compounds to biopharmaceuticals. In vitro data have shown that the application of the device can increase the penetration of angiotensin into the skin 100- fold compared to untreated human skin.
Laser Radiation:
This method involves direct and controlled exposure of a laser beam to the skin which results in the ablation of the stratum corneum without significantly damaging the underlying epidermis. Removal of the stratum corneum using this method has been shown to enhance the delivery of lipophilic and hydrophilic drugs. In 1991, Nelson et al. reported that mid-infrared laser (1 J/cm2) ablation of pig stratum corneum enhanced the permeation of both hydrocortisone and interferon. A handheld portable laser device has been developed by Norwood Abbey Ltd. (Victoria, Australia) that has been approved by the U.S. and Australian regulatory bodies for the administration of a topically applied anesthetic. However, the structural changes caused by this technique still need to be assessed for safety and reversibility, particularly at the higher intensities that may b needed to enhance the penetration of large molecular weight solutes where evidence of deeper level ablation effects exist.
Magnetophoresis:
Magnetophoresis is a novel approach in enhancing drug delivery across biological barriers. Benzoic acid, a diamagnetic substance, was selected as a drug candidate. The influence of magnetic field strength on diffusion flux was determined and was found to increase with increasing applied strength.
REFERENCES:
1. Langer R. Transdermal drug delivery: past progress, current status, and future prospects. Advanced Drug Delivery Reviews 2004; 56: 557-58.
2. Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nature Reviews 2004; 3: 115-24.
3. Jain N, Talegonkar S, Jain N K. New ways to enter the blood stream: Emerging strategies in transdermal drug delivery. The Pharma Review 2004; Oct.: 41- 59.
4. Barry B. Transdermal drug delivery. In: Aulton, E M. (Ed). Pharmaceutics, The science of dosage forms design, ed 2, Churchill Livingstone, Newyork, Harcourt publishers, 2002: 499-33.
5. Kumar P, Sankar C, and Mishra B. Delivery of macromolecules through skin. The Indian Pharmacist 2004; 7-17.
6. Ansel H C, Loyd A V, Popovich N G, Pharmaceutical dosage system, ed 7, Lippincott Williams and Willkins publication
7. Finnin C B. Transdermal drug delivery-What to expect in the near future. Business briefing: Pharma tech. 2003; 192-93.
8. Chien Y W. Transdermal drug delivery and delivery system. Marcel Dekker, Inc. New York, 1992; 50: 301-381.
9. Barry B W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci 2001; 14: 101-14.
10. Gordon RD, Peterson TA. Four myths about transdermal drug delivery. Drug Delivery Technology 3(4): (drugdeliverytech.com/cgibin/ articles.cgi?idArticle=143), June 2003.
11. Access Pharmaceuticals, Inc. EcoNail Fact Sheet. 2011 1-Jan. accesspharma.com/newsroomeconail/ (accessed 2011 26-April).
12. Altea Therapeutics. Altea Therapeutics PassPort Patch. 2010 1-Jan. alteatherapeutics.com/ (accessed 2011 26-April).
13. Badkar, Advait V., et al. “Transdermal delivery of interferon alpha-2B using microporatio









