 About Authors:
About Authors:
Dhirendra C. Patel1*, Ritesh B. Patel1, Gargi B. Patel2
1Department of Pharmaceutics and Pharmaceutical Technology;
S.K. Patel College of Pharmaceutical Education and Research;
Ganpat University, Kherva, Mehsana, Gujarat, India.
2Pharma Management & Regulatory Affairs,
K.B. Institute of Pharmaceutical Education & Research, Gandhinagar, Gujarat, India.
*dhiren.pharmacy@gmail.com
Abstract:
Oral controlled drug delivery systems represent the most popular form of controlled drug delivery systems for the obvious advantages of oral route of drug administration. However, there are certain conditions for which such a release pattern is not suitable like cardiovascular diseases, Diabetes mellitus, Asthma, Arthritis, Peptic ulcer etc. In such cases pulsatile drug delivery system is used in which release drug on programmed pattern i.e. at appropriate time & at appropriate site of action. Pulsatile Drug Delivery systems are basically time controlled drug delivery systems in which the system controls the lag time independent of environmental factors like pH, enzymes, gastro-intestinal motility, etc. The principle rationale for the use of pulsatile release is for the drugs where a constant drug release, i.e., a zero-order release is not desired. In chronopharmacotherapy drug administration is synchronized with biological rhythms to produce maximal therapeutic effect & minimum harm for the patient. Technically, pulsatile drug delivery systems administered via the oral route could be divided into two distinct types, the time controlled delivery systems and the site-specific delivery systems, thus providing special and temporal delivery. In recent pharmaceutical applications involving pulsatile delivery; multiparticulate dosage forms (e.g. pellets) are gaining much favor over single-unit dosage forms. Various pulsatile technologies have been developed on the basis of methodologies, these includes ACCU-BREAK™, AQUALON, CODAS®, PRODAS®, SODAS®, MINITABS®, DIFFUCAPS®, OROS® etc. Designing of proper pulsatile drug delivery will enhance the patient compliance, optimum drug delivery to the target side & minimizing the undesired effects.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1507
INTRODUCTION
Oral controlled drug delivery systems represent the most popular form of controlled drug delivery systems for the obvious advantages of oral route of drug administration. Such systems release the drug with constant or variable release rates. These dosage forms offer many advantages, such as nearly constant drug level at the site of action, prevention of peak-valley fluctuations, reduction in dose of drug, reduced dosage frequency, avoidance of side effects, and improved patient compliance However, there are certain conditions for which such a release pattern is not suitable. These conditions demand release of drug after a lag time. In other words, it is required that the drug should not be released at all during the initial phase of dosage form administration. Such a release pattern is known as pulsatile release.In recent years there is a continuous interest in the development of controlled drug release systems, to achieve the optimal therapeutic effect of drugs. This is based on the increasing awareness of the importance of circadian rhythms with respect to physiology, disease state and drug action which has given rise to the related fields of chronotherapeutics and chronopharmacology. The principle rationale for the use of pulsatile release is for the drugs where a constant drug release, i.e., a zero-order release is not desired. The release of the drug as a pulse after a lag time (an interval of no drug release) has to be designed in such a way that a complete and rapid drug release follows the lag time.
[adsense:468x15:2204050025]
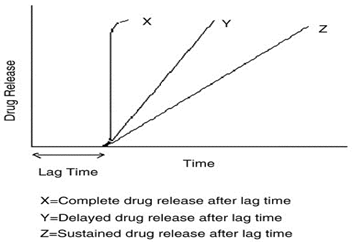
Fig.1: Drug release profile of pulsatile drug delivery system
ADVANTAGES OF PULSATILE DRUG DELIVERY SYSTEM:
· Extended daytime or night time activity.
· Reduced side effects
· Dosage frequency.
· Reduction in dose size.
· Improved patient compliance.
· Lower daily cost to patient due to fever dosage units are required by the patient in therapy.
· Drug adapts to suit circadian rhythms of body functions or diseases.
· Drug targeting to specific sites like colon.
· Protection of mucosa from irritating drugs.
· Drug loss is prevented by extensive first pass metabolism.
DRAWBACKS OF PULSATILE DRUG DELIVERY SYSTEM:
· Lack of manufacturing reproducibility and efficacy
· Large number of process variables.
· Multiple formulation steps.
· Higher cost of production.
· Need of advanced technology.
· Trained/skilled personal needed for manufacturing.
Table 1. Diseases Requiring Pulsatile Delivery
|
Disease |
Chronological behavior |
Drug used |
|
Cardiovascular diseases |
BP is at its lowest during night or at early morning awakening period |
Nitroglycerin, Calcium channel blocker, ACE Inhibitors etc. |
|
Diabetes mellitus |
Increase in the blood sugar level after meal |
Sulfonyl urea, Insulin, Biguanide |
|
Asthma |
Precipitation of attacks during night or at early morning hour. |
Β2 agonist, Antihistaminics |
|
Arthritis |
Pain in the morning and more pain at night |
NSAIDS, Glucocorticoids |
|
Peptic ulcer |
Acid secretion |
H2 blockers |
|
Hypercholesterolemia |
Cholesterol synthesis is generally higher during night than during day time |
HMG CoA reductase Inhibitors |
|
Arthritis |
Pain in the morning and more pain at night |
NSAIDS, Glucocorticoids |
CLASSIFICATION OF PULSATILE SYSTEMS
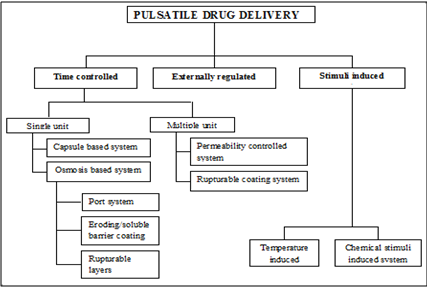
Fig.2: Currently reported classification of pulsatile drug delivery
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
ADVANCED TECHNOLOGY
Currently pharmaceutical company focused on developing and commercializing pulsatile drug products that fulfil unmet medical needs in the treatment of various diseases. For several diseases (e.g. bronchial asthma, hypertension, rheumatic disease and myocardial infarction) as well for control of body functions (blood pressure, levels of many hormones e.g. aldosterone, rennin, and cortisol) influenced by circadian rhythms, delayed or pulsatile drug release could be an optimal approach.Recently develop various technologies that presents in this review are:
1. ACCU-BREAK™ TECHNOLOGY
ACCU-BREAK Pharmaceuticals, Inc. ("ABP" or the Company) is a privately-held pharmaceutical company. ABP has invented and is developing the patent-pending ACCU-BREAK family of technologies (the "Technologies"). ACCU-BREAK tablets are designed to improve and enhance the functionality of pharmaceutical tablets. While being manufactured to be easily swallowed as whole tablets, the Company expects ACCU-BREAK tablets to be Simply Better because in addition, they are Made to be Broken. The ACCU-BREAK innovations are designed to allow pharmaceutical tablets to be able to be easily broken into equal half, third, or quarter doses. Accu-Break tablets are manufactured on commercially available multilayer compression equipment. Accu-Break™ Technology is divided in to two types ACCU-B™ Technology and ACCU-T™ Technology.
ACCU-T CR Trilayer Tablets:
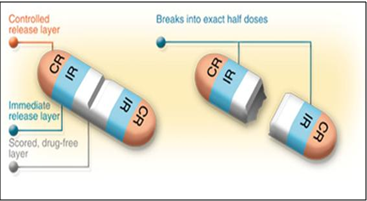
Fig.3: ACCU-T CR Tri-Layer Tablets
ACCU-T CR (controlled release) Tri-Layer Tablets configuration applies controlled release technology to further enhance treatment options. The ACCU-T CR tablet contains controlled release medication at either end of the tablet separated by a drug-free break layer, allowing the CR dose to be divided into exact half doses without affecting the rate of drug release. The majority of conventional CR tablets are not suited for subdividing due to the increase of surface area and the subsequent change in release kinetics. ACCU-T technology provides a solution to this problem and introduces dose flexibility into CR dosage forms. Additionally, an IR (immediate release) component can be added to CR tablets to add even more treatment options and potential product capabilities.
ACCU-B Bilayer Tablets:
A second option is the ACCU-B tablet, which comprises a scored top layer pre-divided into four equal segments, plus an inactive bottom layer, which serves asthe breaking region.
2. AQUALON SYSTEM
Aqualon CMC EZ products are encapsulated to provide ideal performance in dry mixes and in operations with limited mixing/shear capabilities. Hydrocolloids require dispersion before they can fully hydrate and build viscosity. Dispersion methods like high shear mixing and dry blending add time and complexity to operations. This can be minimized by agglomeration and controlling particle size but at the expense of speed of hydration. Aqualon EZ technology offers both easy dispersion and fast hydration. The drug is contained in the inner (middle) layer of the matrix, with the outer layers providing a predetermined delay in release of the drug. A number of studies have been published where tablet cores were coated with directly compressed ethylcellulose (EC), forming a non-swelling, insoluble diffusion barrier to achieve either sustained release or time-controlled, delayed release profiles. Finely powdered (micronized) EC was particularly effective since the smaller particle size renders the material more compatible.
Features and Benefits:
· No lumps
· Easy dispersion significant mixingor highshear is not needed
· Fast hydration – full viscosity in a fraction of the time needed for standard cellulose gum
· Improved performance in dry mix beverage applications
· Ideal for operations where shear and mixing capabilities are limited
3. BANNER’S VERSETROL™ TECHNOLOGY (Patent No: US650045)
Banner is a global polymer based drug delivery and specialty pharmaceutical company. VersetrolTM Technology is novel innovative technology that provides time controlled release for wide range of drug. In this technology drug is incorporated in lipophillic or hydrophilic matrix and that is than incorporated in soft gelatin capsule shell. This technology is versatile because depending on physiochemical properties of drug either emulsion or suspension can be developed. For lipophillic drugs suspension formulation is preferred while for hydrophilic drugs emulsion form is utilized. By applying combination of lipophillic and hydrophilic matrices desire release profile can be achieved. The utility of the Versetrol™ technology is greatly enhanced because it combines a versatile controlled release technology with the benefits of a customer preferred dosage form.
Versetrol controlled release softgels combine the qualities of a consumer preferred dosage form with the ability to control the release rate of active ingredients. Versetrol offers the flexibility to develop almost any release profile by simply tailoring the formulation variables, resulting in a more predictable release profile. Versetrol™ is a unique oral controlled release technology for the pharmaceutical market place. When combined with softgels, controlled release technology provides a consumer preferred dosage form with the ability to tailor release profiles for a wide variety of drugs. Versetrol™ offers controlled release formulations with the benefits of greater effectiveness in the treatment of chronic conditions by reducing side effects through minimizing peak plasma concentrations, and greater convenience leading to higher levels of patient compliance due to a simplified dosage schedule. Versetrol™ technology can be applied early on in pharmaceutical product development, leading to the parallel development of immediate release and controlled release for mutations to maximize market exposure.
Features:
- Unique, proprietary technology
- Novel,innovative controlled releasetechnology system
- Depending onthe physicochemical properties of the compound, an emulsion or suspension is chosen as the formulation
- Varying proportions of hydrophilic and hydrophobic components can be added to the formulation matrix to provide a tailored release profile including a dual release profile
- Good option for moisture sensitive drugs, due to inherent hydrophobic environment in the fill
- Ability to engineer to almost any desired release profile
- Unique matrix formulations for both lipophilic and hydrophilic compounds
- Compatible with EnteriCare® enteric softgels and absorption enhancement techniques
- Consumer preferred dosage form
Benefits:
- Provides first to market opportunity for lifecycle extension and brand building
- Provides market protection for new products
- VersetrolTM technology may be applied to difficult to solubilize compounds
4. CODAS™ TECHNOLOGY (Patent No: US6500459)
In certain cases immediate release of drug is undesirable. A delay of drug action may be required for a variety of reasons. Chronotherapy is an example of when drug release may be programmed to occur after a prolonged interval following administration. Elan’s Chronotherapeutic Oral Drug Absorption System (CODAS™ Technology) was developed to achieve this prolonged interval. Elan’s drug delivery technology can be tailored to release drug after a predetermined delay. The CODAS™ drug delivery system enables a delayed onset of drug release, resulting in a drug release profile that more accurately compliments circadian patterns.
CODAS™ Product Development:
Elan’s Verelan® PM represents a commercialized product using the CODAS™ technology. The Verelan® PM formulation was designed to begin releasing Verapamil approximately four to five hours post ingestion. This delay is introduced by the level of release controlling polymer applied to the drug loaded beads. The release controlling polymer is a combination of water soluble and water insoluble polymers. As water from the gastrointestinal tract contacts the polymer coat beads, the water soluble polymer slowly dissolves, and the drug diffuses through the resulting pores in the coating. The water insoluble polymer continues to act as a barrier, maintaining the controlled release of the drug. When taken at bedtime, this controlled onset extended release delivery system enables a maximum plasma concentration of Verapamil in the morning hours, when blood pressure normally rises from its overnight low.
5. COLAL TECHNOLOGY (Patent No: WO03068196)
COLAL® involves a coating for drug pellets, tablets or capsules which is composed of ethylcellulose and a form of starch called 'glassy amylose'. The glassy amylose is not digested by human enzymes as the preparation moves down the GI tract, but is digested by bacterial enzymes that are found only in the colon. When the coated product reaches the colon, the coating is degraded, allowing the drug to be released. This technology useful for to Treat Ulcerative Colitis administered Directly to the Colon Completed Phase III Development.In ulcerative colitis, inflammation occurs in areas of the colon and rectum causing pain, diarrhoea and bleeding. Current treatment includes an aggressive therapy for short periods with an antiinflammatory steroid (usually prednisolone) to induce remission of the disease and the use of less potent antiinflammatories over longer periods for the maintenance of the disease in remission. However, the use of anti inflammatory steroids is restricted by their systemic side effects. The worldwide market for ulcerative colitis drugs is estimated to be worth at least $500 million per annum. A product with a substantially improved safety/efficacy profile over existing treatments would be clinically and commercially attractive.
COLAL® comprises a prednisolone derivative (prednisolone sodium metasulphobenzoate) in Alizyme's proprietary colonic drug delivery technology, COLAL®. Data from Alizyme's Phase II trial with COLAL®, indicated that through the local delivery of this drug, the undesirable side effects such as immuno suppression, associated with the use of steroids are avoided.
6. DIFFUTAB® TECHNOLOGY (Patent No: US650045)
Multiparticulate drug dosage forms are composed of small beads, each small bead further composed of many layers. Some layers contain drug substance; others are rate controlling polymers. With Eurand’s Diffucap® multiparticulate system, customized drug release profiles are created by first layering active drug onto an inert core (such as a cellulose sphere), then applying one or more rate controlling, functional polymers, to produce spherical, multilayered particles. The drug layering process can be conducted either from aqueous or solvent based drug solutions. Diffutab® technology work on same principle as given below;
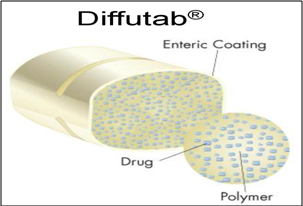
Fig.4: Diffutab® technology
Diffutab® technology for sustained release profiles and targeted delivery of pharmaceutical products. This technology incorporates a blend of hydrophilic polymers that control drug release via diffusion through, and erosion of, a matrix tablet. The Diffutab technology is particularly useful for the development of high dosage products and is an effective way to develop sustained release, once a day dosage forms.
Advantages:
- Matrix tablet utilizes a combination of water soluble particles and active drug
- Suitable for high drug loading
- Supports sustained release, once a day dosing
7. DMDS TECHNOLOGY
DMDS (Dividable Multiple Action Delivery System) is designed to provide greater dosing flexibility that improve product efficacy and reduces side effects. Traditional controlled release tablet often lose their controlled release mechanism of delivery once it broken. But DMDS technology allows tablet to be broken down in half so that each respective portion of the tablet will achieve exactly the same release profile as the whole tablet. This allows the patient and physician to adjust the dosing regimen according to the clinical needs without compromising efficacy.
8. EGALET®TECHNOLOGY
Egalet has pioneered one of the world’s first erosion based delivery technologies to enable the controlled release of drugs through gradual erosion of a tablet. In addition, the Egalet® technology, a novel, patented drug delivery platform, was designed to resist tampering, to prevent easy extraction and to deter the abuse of medications via known routes of abuse, including chewing, snorting, and injecting. The uniquely shaped, patient friendly tablet consists of matrix and can add a shell or coat. By altering the composition of the shell and matrix, a variety of release formulations can be produced. The technology offers a predictable and tailored pharmacokinetic profile, lacks a significant food effect and alcohol dose dumping, and can be used with a broad range of opioids and nonopioids. Egalet has extensively filed patents to protect its inventions covering both the technology and product specific patents. The Egalet® Time Release consists of three compartments: a coat, a drug release matrix and a lag component. The drug is contained in the inner (middle) layer of the matrix, with the outer layers providing a predetermined delay in release of the drug.
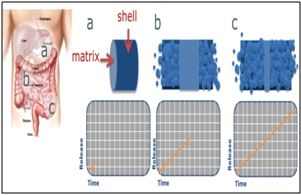
Fig.5: Egalet® Two-Part Oral System
1. Egalet tablet begins
2. Egalet tablet during release
3. Egalet tablet has released almost completely
9. EURAND MINITABS® TECHNOLOGY
Eurand Minitabs® is a registered trademark used for Pharmaceutical Carrier Preparation with Sustained Release Properties. Eurand Minitabs® technology combines the simplicity of tablet formulation with the sophisticated drug release control offered by multiparticulate drug forms. Eurand Minitabs are tiny, approximately 2 mm in diameter, cylindrical tablets. Functional membranes may be applied to the tablets to further control release rate. Eurand Minitabs offer high drug loading, a wide range of release rate designs, and fine tuning of these release rates. Capsules containing the Eurand Minitabs can be opened and the contents used as a “sprinkle” formulation. The tablets are filled into capsules, allowing a combination of multiple drugs and/or multiple release profiles in the same dosage form. The Eurand Minitabs® can be formulated as matrix tablets prior to further coating.
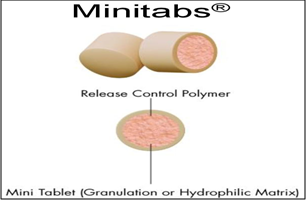
Fig.6: Eurand minitabs® technology
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Advantages:
· Combines the simplicity of tablet formulation with the sophistication of multiparticulate systems
· Suitable for high drug loading
· Can be used as a sprinkle for pediatric and geriatric patients who have difficulty swallowing tablets
10. EURAND’S DIFFUCAPS®MULTIPARTICULATE SYSTEM
Diffucaps technology facilitates the development and commercialization of novel, controlled release delivery systems for once or twice daily dosing of single drugs or drug combinations that exhibit extreme pH dependent solubility profiles and/or are poorly soluble in physiological fluids.This multiparticulate system can provide dosage strength flexibility, and required pK profile and optimal release profiles for single drugs and drug combinations. The Diffucaps® drug release system can also be used in combination with other proprietary technologies to enhance drug solubility in the GI tract. Diffucaps is a multiparticulate bead system comprised of multiple layers of drug, excipients, and release controlling polymers. The beads contain a layer of organic acid or alkaline buffer to control the solubility of a drug by creating an optimal pH microenvironment for drugs that exhibit poor solubility in intestinal pH, in environments with pH greater than 8.0, or in physiological fluids. Alternatively, the beads can contain a solid solution of drug and crystallization inhibitor to enhance bioavailability by maintaining the drug in its amorphous state.
Diffucaps technology is especially suitable for drugs that traditionally require multiple daily doses or drugs needing customized release formulations. Each Diffucaps bead has an inert core surrounded by drug and coated with a functional polymer membrane to control the rate of drug release.
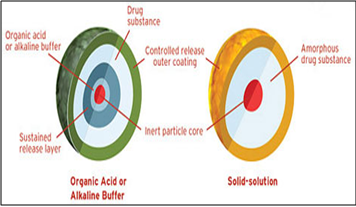
Fig.7: Eurand’s Diffucap technology
Advantages of Diffucaps
- Ideal for drugs exhibiting poor solubility in lower intestinal pH, in environments with pH above 8.0, or in physiological fluids
- Can combine multiple drugs and/or multiple release profiles in the same dosage form
- Simple formulation of dose proportional strengths.
- Can minimize food effect
11. EURAND’S PULSATILE & CHRONO RELEASE SYSTEM
Eurand’s Time controlled pulsatile release system is capable of providing one or more rapid release pulses at predetermined lag times, such as when Chronotherapy is required, and at specific sites, such as for absorption along the GI tract. These capabilities can help optimize efficacy and/or minimize side effects of a drug substance. For example, Eurand has created a circadian rhythm release (CRR) dosage form for a cardiovascular drug, Propranolol hydrochloride, with a four hour delay in release after oral administration. Administered at bedtime, Propranolol is released after the initial delay such that maximum plasma level occurs in the early morning hours, when the Patient is most at risk.
12. GEOCLOCK®TECHNOLOGY
Combining formulation skills with controlled manufacturing processes, SkyePharma developed a new oral drug delivery technology, Geoclock®; that allows the preparation of Chronotherapy focused press coated tablets. Geoclock® tablets have an active drug inside an outer tablet layer consisting of a mixture of hydrophobic wax and brittle material in order to obtain a pH independent lag time prior to core drug delivery at a predetermined release rate. This dry coating approach is designed to allow the timed release of both slow release and immediate release active cores by releasing the inner table first after which time the surrounding outer shell gradually disintegrates. As well as controlled release, the Geoclock® technology also has applications for the improved release of colonic drug delivery, as well as multiple pulse drug delivery to deliver doses of the drug at specific times throughout the day.
Using this novel technology, SkyePharma has been developing Lodotra™, a rheumathoid arthritis drug, on behalf of Nitec Pharma. Lodotra™ will deliver the active pharmaceutical ingredient at the most suitable time of day to treat the disease.
13. GEOMATRIX™ TECHNOLOGY
The Geomatrix™ technology is applied to achieve customised levels of controlled release of specific drugs and can achieve simultaneous release of two different drugs and different rates from a single tablet. The controlled release is achieved by constructing a multilayered tablet made of two basic key components;
A. Hydrophilic polymers such as hydroxypropyl methylcellulose (HPMC) and
B. Surface controlling barrier layers.
Active loaded core surface that is available for drug release when exposed to the fluid is controlled by barrier layers. The combination of layers, each with different rates of swelling, gelling and erosion, is responsible for the rate of drug release within the body. When first swallowed, for example, the drug concentration is high but the surface area low. As time progresses the core swells and the surface area increases to compensate for the decrease in drug concentration. One of the major benefits of the Geomatrix™ technology is its ability to be easily incorporated into the production line. The Geomatrix™ tablets can be manufactured by readily available equipment that can be integrated into widely used pharmaceutical processes, thus giving firms more control over their own production activities.
Advantages
· Reproducibility
· Efficacy
· Versatility of release control mechanisms
· Controlled release of poorly soluble drugs
· Timed release of drugs
· Biphasic release of drugs
· Release of 2 or more drugs at different rates
· Pulsed release of drugs
14. INTELLIMATRIX™ TECHNOLOGY
Intelli Pharmaceutical is a pharmaceutical technology development company with a suite of proprietary tablet technologies. This Company have developed Novel oral Time controlled Release Matrix tablet Known as IntelliMatrixTM tablet. IntelliMatrixTM drug delivery platform is unique composition of several different ‘intelligent’ polymers such as hydroxy ethylcellulose and a channel former as Lactose. IntelliMatrixTM system is at the heart of proprietary drug delivery. Proprietary modelling enables precise profile control and site specific drug delivery.
15. IPDAS® TECHNOLOGY
The Intestinal Protective Drug Absorption System (IPDAS® Technology) is a high density multiparticulate tablet technology, intended for use with GI irritant compounds. IPDAS®, Intestinal Protective Drug Absorption System, was initially developed as part of the development process for Elan’s proprietary naproxen formulation, Naprelan®. The objective was to develop a once daily controlled release system that would have a fast onset of action and reduced gastric irritancy. IPDAS® delivery system can also be employed to confer the advantages of multiparticulate technology, in a tablet dosage form. The IPDAS® technology is composed of numerous high density controlled release beads, which are compressed into a tablet form. Once an IPDAS® tablet is ingested, it rapidly disintegrates and disperses beads containing a drug in the stomach, which subsequently pass into the duodenum and along the gastrointestinal tract in a controlled and gradual manner, independent of the feeding state. Release of active ingredient from the multiparticulate occurs through a process of diffusion either through the polymeric membrane and or the micromatrix of polymer/active ingredient formed in the extruded/spheronized multiparticulate. The intestinal protection of IPDAS® technology is by virtue of the multiparticulate nature of the formulation, which ensures wide dispersion of irritant drug throughout the gastrointestinal tract.
16. MAGNETIC NANOCOMPOSITE HYDROGEL
Magnetic nanocomposite of temperature responsive hydrogel was used as remote controlled pulsatile drug delivery. Nanocomposites were synthesized by incorporation of superparamagnetic Fe3O4 particles in negative temperature sensitive poly (N-Nisopropylacrylamide) hydrogels. High frequency alternating magnetic field was applied to produce on demand pulsatile drug release from nanocomposite hydrogel. Nanocomposite hydrogels temperature increase above LCTS so, result in to accelerated collapse of gel. Hence Nanocomposite hydrogels are one type of On-Off device where drug release can be turn on by application of alternative magnetic field.
Hydrogels are three dimensional polymeric networks with the ability to swell several times their dry weight by absorption of water and other biological fluids. Hydrogels are currently considered for numerous biomedical and pharmaceutical applications including drug delivery devices, contact lenses, tissue engineering scaffolds, biosensors, sutures, and components of microfluidic devices.Responsive hydrogels are a class of hydrogels with swelling properties dependent on environmental factors like pH, temperature, ionic strength, and the presence of a particular molecule.
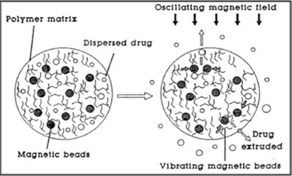
Fig.8: Magnetic Nanocomposite hydrogels
17. OPANA® ER TECHNOLOGY
OPANA ER is indicated for the relief of moderate to severe pain in patients requiring continuous, around the clock opioid treatment for an extended period of time. Since its approval in 2006, Opana® ER has been used to treat patients with moderate to severe chronic pain from conditions such as chronic lower back pain and osteoarthritis. The Opana ER tablet has gone through some changes. In 2011, Opana ER was available in a tablet shape that was “biconvex,” or raised and rounded on both sides. There may be limited quantities of both the biconvex tablets of Opana ER and the octagonal original formulation tablets available through your local pharmacy. Moving forward, the tablet shape will be "biconcave," or indented on both sides. While the tablet may look different, it’s still the same medicine.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
18. ORBEXA® TECHNOLOGY
Orbexa® technology is a multiparticulate system that enables high drug loading and provides a formulation choice for products that require granulation. This technology produces beads that are of controlled size and density and suitable for formulation as controlled release multiparticulate using granulation, spheronization and extrusion techniques. The resultant beads can be coated with functional polymer membranes for additional release rate control and may be filled into capsules or provided in sachet form.
Advantages:
· Aqueous or solvent based granulation
· High speed process is well suited for sensitive molecules like proteins
· Suitable for high drug loading
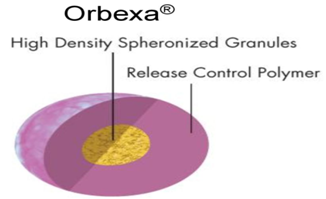
Fig.9: ORBEXA® Technology
19. OROS TECHNOLOGY
OROS R technology uses an osmotic mechanism to provide pre-programmed, controlled drug delivery to the gastrointestinal tract. The dosage form comprises a wall that defines a compartment. The active drug is housed in a reservoir, surrounded by a semipermeable membrane/wall (e.g. cellulose esters, cellulose ethers and cellulose ester–ethers) and formulated into a tablet. The tablet is divided into two layers, an active drug layer and a layer of osmotically active agents (e.g. poly (ethylene oxide)) comprising means for changing from a non dispensable viscosity to a dispensable viscosity when contacted by fluid that enters the dosage form.
OROS delivery systems were adopted for poorly water soluble drugs. The push-pull system is comprised of a bilayer or trilayer tablet core consisting of one push layer and one or more drug layers. The drug layer contains the poorly soluble drugs, osmotic agents and a suspending agent. The push layer contains among other things, an osmotic agent and water swellable polymers. A semipermeable membrane surrounds the tablet core. A variety of OROS® systems (ALZA Corp.) have been developed: Procardia XL®, Ditropan XL®and Concerta® are notable examples. The recently developed L-OROS® SOFTCAPTM delivery system combines the features of a controlled release and bioavailability enhanced delivery system to enhance compliance and therapeutic effect.66,67
Controlled Release of Non Aqueous Liquid Formulation
· L-OROS Hard cap
· L-OROS Soft cap
The liquid drug formulation is encased in soft capsule. It is in turn surrounded by a barrier layer, osmotic engine, and a semi permeable membrane in order. A delivery orifice in drilled through semipermeable membrane, osmotic engine and barrier layer.
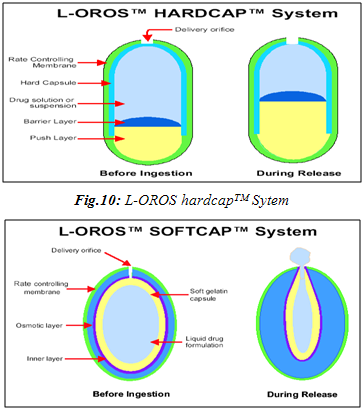
Fig.11: L-OROS softcapTM Sytem
Advantages:
· Enhanced bioavailability of class II drugs
· Uniform blood levels over specific period of time
· Reduced first pass effect
· Reduced dose
· Patient compliance
· Made of pharmaceutical acceptable excipient
20. OSDrC® TECHNOLOGY (Patent No: WO0198067)
OSDrC® means one step dry coating Technology. This Technology opens the door to new world of pharmaceutical tablet manufacturing. The key word in this new world is “unique”, “High quality”, “low cost” and “Innovative”. OSDrC® optimized dose delivery technology enables the design of single or multi-core tablets, with a practically endless variety of core numbers, shapes, sizes, and placement within the tablet. This flexible-core capability offers the broadest available range of controlled release designs for drug formulators, providing the potential for optimal dosing, therapeutic product profiles, and plasma release profiles to meet patient needs in a high quality, one step manufacturing process.
Advantages of this technology:
· Accurate & Flexible Control Technology: OSDrC® technology allows placement of any number of cores of any shape into the tablet just where they need to be positioned for optimum delivery of active pharmaceutical ingredients (API). Misaligned cores are a thing of the past. This paves the way for high value-added drug formulation development, such as divided tablets with two cores, pulsatile tablets with three cores, and combination products.
· Poor-Compressibility Encasing Technology: OSDrC® technology allows incorporation of core ingredients with poor compressibility, such as magnesium stearate. OSDrC® tablets with pellets as their core can replace conventional capsules. OSDrC® paves the way for development of various novel drug formulations, such as new oral rapid disintegration tablets.
· OSDrC® Provides Controlled Release: Precise OSDrC® positioning technology enables product development scientists to control the release of the API by altering the thickness of the outer coating. The ability to precisely position multiple cores allows the creation of tablet products with a variety of pulsatile drug release profiles.
· One-Step Dry-Coating Technology: The OSDrC® rotary tableting machine, with its variable double-punch configuration, supports single-step manufacturing of pharmaceutical products. In addition to the commercial-scale production of conventional cored (tablet-within-a-tablet) tablets, this machine is ideal for manufacturing a variety of high-quality drug products at low cost. This innovative technology can also replace conventional sugar- and film-coated tablets.
21. PMDS® TECHNOLOGY
PMDS® (Programmed Multiple action Delivery System) technology is designed to provide for the multiphasic delivery of any active ingredient in a more controlled fashion as compared to typical controlled release technologies. This system controls release rates for multiple ingredients within a single table in a programmed manner. Our TMDS technology allows for the release of more than one active ingredient in a single tablet formulation to be released in multiple profiles over time. PMDS technology is designed to allow for the release of the active ingredient at predetermined time intervals and desired levels on a consistent basis. This technology allows us to overcome one of the technical challenges in the development of multi-particulate dosage forms achieving acceptable uniformity and reproducibility of a product with a variety of release rates. It is designed to provide greater dosing flexibility that improves product efficacy and may reduce side effects.
22. PORT TECHNOLOGY
A water permeable coated gelatin capsule with osmotic core that swells and is sealed with an insoluble wax plug. The content swells to remove the plug. The wall thickness and composition, concentration of the osmotic contents and the length of the hydrogel plug control lag time. Ritalin (methyl phenidate) used in the treatment of attention deficit hyper active disorder (ADHD) in children, is formulated as PORT system. The use of this system avoided second time dosing which is beneficial for school children.
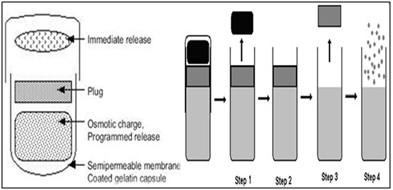
Fig.12:Port technology
· Step1: Cap dissolves off. Immediately or modified release dose is released.
· Step 2: Energy source is activated by controlled permeation of GI fluid.
· Step 3: Time-release plug is expelled.
· Step 4: Pulse or Sustained release of second dose.
23. PRODAS® TECHNOLOGY (Patent No: US650045)
Programmable Oral Drug Absorption System (PRODAS® Technology) is a multiparticulate technology, which is unique in that it combines the benefits of tabletting technology within a capsule. The PRODAS® delivery system is presented as a number of minitablets combined in a hard gelatine capsule. Very flexible, the PRODAS® technology can be used to pre-program the release rate of a drug. It is possible to incorporate many different minitablets, each one formulated individually and programmed to release drug at different sites within the gastro-intestinal tract. It is also possible to incorporate minitablets of different sizes so that high drug loading is possible. PRODAS® technology, by incorporating minitablets with different release rates, can display the characteristics of a number of different conventional dosage forms:

Fig.13:PRODAS® Technology
24. PULSINCAPTM TECHNOLOGY(Patent No: US5631022)
Pulsincap(R) is an oral drug delivery device which is designed to release the drug in a pulsed fashion at a predetermined time in the gastrointestinal tract or at a predetermined site in the body. This dosage form consists of a capsule composed of a water insoluble body and a water soluble cap. The drug formulation is contained within the capsule body and is sealed in by a hydrogel plug. At a specified time after ingestion, the drug is released into the small intestine or colon for absorption into the blood stream. The plug material consists of insoluble but permeable and swellable polymers (eg, polymethacrylates), erodible compressed polymers (eg, hydroxypropylmethyl cellulose, polyvinyl alcohol, polyethylene oxide) (Krögel et al., 1998), congealed melted polymers (eg, saturated polyglycolated glycerides, glyceryl monooleate), and enzymatically controlled erodible polymer (eg, pectin, agar) (Krögel et al., 1999).
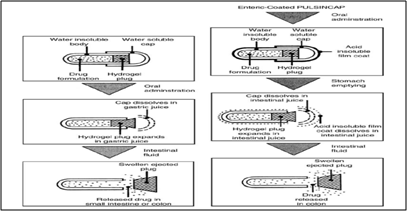
Fig.14: PULSINCAPTM TECHNOLOGY









